Comparative Analysis of Andiroba Oil obtained by Artisanal and Commercial Process: Fatty Acid Profile and Microencapsulation
DOI 10.5433/1679-0375.2024.v45.51534
Citation Semin., Ciênc. Exatas Tecnol. 2024, v. 45: e51534
Received: 1 October 2024 Received in revised for: 12 November 2024 Accepted: 27 November 2024 Available online: 19 December 2024
Abstract:
This study aimed to evaluate how the extraction method of andiroba oil interferes with the lipid composition and formation of microcapsules, obtained through the complex coacervation technique. Chromatographic analysis and quantification of triacylglycerols in commercial and artisanal andiroba oils showed high values for saturated and monounsaturated fatty acids, with the commercial oil showing a higher content of polyunsaturated fatty acids. Microencapsulation did not affect the lipid profile of the evaluated oils. Optical and scanning electron microscopy revealed multinucleate microcapsules with well-defined walls, which may provide greater protection to the nucleus. The type of oil used in the formulations affected the size distribution of the microcapsules, with commercial oil yielding more homogeneous microcapsules, and low polydispersity value. The high encapsulation efficiency was also observed in commercial oil microcapsules, demonstrating that the origin of the nucleus is important for the formation of better quality microcapsules.
Keywords: alginate, essential oil, gelatin, microcapsules, complex coacervation
Introduction
Carapa guianensis is a large neotropical tree belonging to the Meliaceae family, found in northern South America, Central America, the Caribbean and sub-Saharan Africa. In Brazil, it is known as andiroba, originating in floodplains and flooded areas throughout the Amazon region (Costa-Silva et al., 2008).
Practically all parts of Carapa guianensisare utilized, especially the oil, extracted from the seed, which is used both in industry and in research, in various applications, such as anti-allergic, repellent, anti-fungal, and anti-inflammatory (Ambrozin et al., 2006). These biological activities are due to its composition, mostly, by triacylglycerols with elevated levels of unsaturated fatty acids, such as oleic, palmitic, stearic and linoleic; in addition to limonoids, triterpenes, steroids, coumarins, flavonoids and diglycerides (Cabral et al., 2013). Among these compounds, limonoids stand out, which have an insecticidal effect and are produced by plants as a defense mechanism against phytophagous insects (Senhorini et al., 2012).
The purposes attributed to andiroba have driven the oil processing, adding value to the final product, and enhancing its functionalities (Sousa et al., 2019). The choice of extraction method directly affects the quality and quantity of the final product, such as the content of fatty acids present in the oil.
The artisanal oil extraction process is carried out by riverside communities in the Amazon region and consists of cooking the seeds and leaving them to rest for a few days. After this process, the seeds are manually kneaded and again left to rest, for the gradual release of the oil, by dripping, followed by storage in dark glass jars (Brito et al., 2020).
Commercial extraction is done by cold pressing, where the seeds are broken into small pieces, which are placed in a drying oven at 60-70 °C, until reaching 8% moisture. The seeds are then pressed by hydraulic presses, and the extracted oil is centrifuged to remove residues, bottled, and sold (Souza et al., 2006).
The final production of the oil is intended for personal consumption or for commercialization, considered one of the best-selling natural remedies in the Amazon, available at fairs, pharmacies and by independent sellers (Shanley & Londres, 2011). This product serves as an input for the cosmetic, pharmaceutical, food and textile industries, being part of the composition of perfumes, personal hygiene and beauty products, dyes and functional foods (Brito et al., 2020).
However, Andiroba oil, like all essential oils, has limitations in its use, due to volatility, heat sensitivity and oxidation (Matos et al., 2018). Thus, the microencapsulation technique can be an alternative to increase the stability of andiroba oil, during the processing of a product, providing external protection against possible oxidation processes, extending the shelf life, in addition to providing a controlled release of the active ingredients (Senhorini et al., 2012).
Complex coacervation microencapsulation is a widely used technique to microencapsulate lipophilic compounds, such as essential oils. The microcapsules produced by this method have excellent controlled release characteristics, heat resistance properties and high encapsulation efficiency (Ziming Yang et al., 2014).
The polymeric combinations gelatin/gum arabic are the most widely used encapsulating agents in the complex coacervation technique. Coacervates are formed when a dilute mixed solution of gelatin and gum arabic is brought to a pH where the polyelectrolytes have opposite net charges. Under these conditions, the solution separates into a highly concentrated phase, a dry phase in the form of microparticles and a dilute bulk phase (Shaddel et al., 2018). However, other materials have been studied, such as proteins (casein, albumin, etc.) and carbohydrates (alginate, maltodextrin, xanthan gum, high and low methoxylated pectins, carrageenan, among others). For each polymer pair chosen, the process conditions must be studied and established (De Kruif et al., 2004; Souza et al., 2006).
A variable that must also be considered in the microencapsulation process by complex coacervation is the composition of active ingredient that can interfere with the characteristics of the microcapsule formed, such as particle size and morphology (Hijo et al., 2022). In this context, the objective of this study was to how the extraction method of andiroba oil interferes with the lipid composition and formation of microcapsules, obtained by the complex coacervation technique.
Materials and methods
Sampling
The artisanal andiroba oil (AO) was obtained from rural producers, in an open market in the municipality of Codajás (3º 50’ 13’’ S 62º 03’ 25’’ W), Amazonas, Brazil. Commercial andiroba oil (CO), branded Pharmakos D’Amazônia, was acquired in the regional market of Manaus, Amazonas, Brazil.
The microencapsulation biopolymers used were sodium alginate (Inlab, Alamar Tecno-Científica Ltda. Brazil) and bovine gelatin (240 bloom Gelita, Maringá, Brazil). For the microencapsulation process by complex coacervation, Milli-Q ultrapure water (Elga, Purelab Option-Q, Brazil) and glacial acetic acid (Dinâmica, PM 60.05, Brazil) were used for pH adjustment.
Fatty acid composition of andiroba oil by gas chromatography associated with a flame ionization detector (GC-FID)
The characterization of the fatty acid profile of artisanal (AO) and commercial (CO) andiroba oils was performed according to the methodology of Pizzo et al., 2019.
Fatty acid methyl esters (FAMEs) from lipid extracts were prepared by methylation of total lipids, according to International, 2000. In micro tubes, containing 100 mg of lipid sample, 2 mL of heptane were added, and stirred for 2 minutes in a magnetic stirrer (Fisatom mod: 653, Brazil). Then, the material was transferred to a test tube, and 2 mL of reagent-esterifying KOH in methanol (2 mol.L\(^{-1}\)) were added. The solution was vortexed for 3 minutes and refrigerated for 24 hours for phase separation.
The supernatant was collected and transferred to vials for chromatographic analysis, in a Thermo Scientific (GC) chromatograph, equipped with a flame ionization detector (FID), split/splitless injector, and a capillary column (CP-7420 fused silica Select FAME, 100.0 m long, 0.25 mm internal diameter and 0.25 µm cyanopropyl film as stationary phase). The operating parameters were as follows: column temperature of 165 ºC for 18 min, then heated to 235 ºC (4 ºC min-1) for 20 min. Injector and detector temperatures were maintained at 230 and 250 ºC, respectively. The gas flows were 1.2 mL min-1 for the carrier gas (H2), 30.0 mL min-1 for the replacement gas (N2), and in the FID detector 30.0 and 300.0 mL min-1 of H2 gas and synthetic air, respectively. The dimensions were injected in split mode, with a 1:40 ratio. The injection volume was 1.0 µL. FAMEs were identified by comparing the retention times of sample constituents with those of analytical standards (FAME standard mixture, C4-C24, Saint Louis, USA, Sigma-Aldrich). Peak areas were determined using ChromQuest 5.0 software, and fatty acid compositions were expressed as a relative percentage of total fatty acid. All samples were analyzed in triplicate.
The fatty acid profile of the microencapsulated oils was also determined. Oil extraction from the microcapsules (wet base) was performed using the lipid extraction methodology of Bligh & Dyer, 1959. After extraction, the oils obtained were placed in tubes for further analysis.
Triacylglycerols (TAG) in andiroba oil
Based on the fatty acid composition, the TAG ions present in andiroba oil samples were estimated. For the estimation, we used the LAMES Platform ([UFG_n_d - missing information]),
which is based on the mathematical algorithm that describes the distribution of fatty acids in TAG molecules (Antoniosi et al., ), using the percentage of fatty acids, determined by GC-FID. With the Lipid Maps database, it was possible to find putative molecular formulas for the TAGs.
Microcapsule preparation
The preparation of alginate/gelatin microcapsules was carried out according to the methodology adapted from Marfil et al., 2016. First, andiroba oil (2 g) was added to an aqueous gelatin solution (30 g/L), and homogenized in a mechanical stirrer (Fisatom, model 713, Brazil), for 5 minutes at 10,000 rpm and room temperature (25 °C). Then, the solution was heated to \(50 \pm 3\) ºC, and a polymeric solution of alginate (10 g/L) was added, homogenizing for 3 minutes, at 10,000 rpm. Subsequently, the pH was adjusted to \(3.5 \pm 0.3\) with glacial acetic acid. Then, the solution was immersed in an ice bath for slow cooling to \(10 \pm 2\) ºC. At last, the solution was properly packaged, protected from light, and stored for 24 hours in a refrigerator, at 5 ºC, for microcapsules sedimentation.
The coacervate was separated from the solution by filtration on filter paper, placed in plastic pots, and stored in a freezer for further analysis. Two microcapsule formulations were prepared: one containing artisanal oil (MAO) and the other containing commercial oil (MCO).
Determination of moisture, encapsulation efficiency and loading capacity xxxxx
The moisture of the microcapsules was evaluated according to the methodology described by Association of Official Analytical Chemists AOAC/2005 (Association of Official Analytical Chemists [AOAC], ), in an oven at 105 °C, until constant weight. This analysis was performed in triplicate.
To determine the encapsulation efficiency, about 0.3 g of sample were added to tubes, containing 5 mL of isopropanol, 2 mL of distilled water and 2 mL of hexane. Then, the tubes were vortexed and centrifuged at 8000 rpm for 15 minutes (Fayad et al., 2015). After phase separation, the aqueous phase was discarded and the organic phase, containing oil released from the microcapsule, was placed in Petri dishes, and dried in an oven at 25 ºC for 24 hours. The dry material was then weighed on an analytical scale (\(M_{f~\text{oil}}\)), to calculate the encapsulation efficiency and loading capacity. Samples were analyzed in duplicate.
The theoretical value of microencapsulated andiroba oil (\(OT\\)) and the loading capacity (\(LC\\)) were calculated using equations (1) and (2), respectively: \[ OT (\%) = \frac{M_{i~\text{oil}}}{M_{i~\text{microcapsule}}} \times 100,\]
\[ LC (\%) = \frac{M_{f~\text{oil}}}{M_{f~\text{microcapsule}}} \times 100,\]
\(M_{i~\text{oil}}\) is the initial mass of andiroba oil to the system (g), \(M_{i~\text{microcapsule}}\) is the initial mass of wall material (g), \(M_{f~\text{oil}}\) is the oil content after microencapsulation (g), and \(M_{f~\text{microcapsule}}\) is the final mass of product after microencapsulation (g). All calculations were performed on a dry basis.
Encapsulation efficiency (\(EE\)) is given by equation (3)
\[ EE (\%) = \frac{LC (\%)}{OT (\%)} \times 100.\]
Optical microscopy of microcapsules
The morphology of the outer surface of the microcapsules was evaluated by optical microscopy, according to the methodology Marfil et al., 2016. Wet samples were placed directly on slides and covered with a cover slip. An optical microscope (Olympus CX31, Japan) equipped with a SC30 camera, equipped with Analysis getlT software was used to obtain the images. Samples were observed with 20x and 30x objectives.
Scanning electron microscopy (SEM) of microcapsules xxxxxxxxxxxxxx
To study the morphology of the microcapsules by SEM, the samples were frozen followed by lyophilization in a benchtop lyophilizer (Christ, Alpha 1-4 LD, Germany) for 24 hours. The samples were placed on a carbon tape and metallized with a thin layer of gold and palladium in a metallizer (Baltec SCD 050) and evaluated using a scanning electron microscope (Fei Quanta 250, Japan), with an accelerating voltage of 15 kV (Araújo et al., 2020).
Analysis of distribution and mean diameter of microcapsules xxxxxxxxxxxxx
The analysis of the distribution and the average size of the artisanal and commercial microcapsules, in suspension, was carried out at 25 °C, using a Litesizer 500 equipment (Anton Paar, USA), which uses the dynamic light scattering (DLS) technique. This technique is based on the principle that a particle exhibits Brownian motion when suspended. Its size can be determined using the Stokes-Einstein correlation, which relates the diffusion coefficient and the hydrodynamic radius(Amoroso et al., 2020; Pillai & Mandal, 2020).
The microcapsules were suspended in distilled water, the quantities being adjusted to an adequate level of obscuration for the detection of the equipment, being submitted to three readings. The size distribution of the dispersions (D10, D50, D90, corresponding to the diameters referring to 10, 50 and 90% of the accumulated distribution), average diameter and the polydispersity (Span) were generated by the equipment itself. The polydispersity of a particle size distribution was used to express the degree of uniformity.
Statistical analysis
Data were subjected to analysis of variance (ANOVA) and Tukey’s test of means at 5% (p<0.05). All treatments were submitted to the ASSISTAT statistical program (Version 7.6, DEAG-CTRN-UFGC, Campina Grande, Paraíba, Brazil).
Results and discussion
Fatty acid profile of andiroba
The fatty acid profiles present in artisanal and commercial andiroba oil, in addition to the microencapsulated lipid extracts of these oils, were evaluated by GC-FID, Table 1. In all samples, the major fatty acids identified were oleic (C18:1n-9), palmitic (C16:0), stearic (C18:0) and linoleic (18:2n-6) acids.
| Structure | Name | AO | CO | MAO | MCO |
|---|---|---|---|---|---|
| \(10:0\) | Capric acid | \(0.19^a\pm0.04\) | ND | \(0.17^a\pm0.00\) | ND |
| \(12:0\) | Lauric acid | \(0.02^a\pm0.00\) | ND | \(0.01^a\pm0.00\) | ND |
| \(14:0\) | Myristic acid | \(0.04^b\pm0.01\) | \(0.04^b\pm0.00\) | \(0.09^a\pm0.00\) | \(0.02^c\pm0.04\) |
| \(16:0\) | Palmitic acid | \(28.51^a\pm1.08\) | \(27.28^a\pm0.12\) | \(28.20^a\pm0.02\) | \(27.29^a\pm0.02\) |
| \(16:1n-9\) | Hexadecenoic acid | \(0.48^b\pm0.03\) | \(0.35^d\pm0.02\) | \(0.86^a\pm0.02\) | \(0.42^c\pm0.01\) |
| \(17:0\) | Margaric acid | \(0.09^a\pm0.00\) | \(0.08^a\pm0.00\) | \(0.10^a\pm0.01\) | \(0.09^a\pm0.01\) |
| \(18:0\) | Stearic acid | \(7.08^c\pm0.12\) | \(7.44^b\pm0.20\) | \(7.36c\pm0.09\) | \(7.88^a\pm0.00\) |
| \(18:1n-9\) | Oleic acid | \(54.19^{ab}\pm0.87\) | \(54.65^a\pm0.20\) | \(53.06^c\pm0.15\) | \(54.72^a\pm0.07\) |
| \(18:1n-7\) | Vacenic Acid | \(0.92^a\pm0.19\) | \(0.77^a\pm0.08\) | \(1.01^a\pm0.02\) | \(0.06^b\pm0.01\) |
| \(18:2n-6\) | Linoleic acid | \(6.79^b\pm0.11\) | \(7.79^a\pm0.17\) | \(7.68^a\pm0.08\) | \(7.82^a\pm0.00\) |
| \(20:0\) | Arachidic acid | \(0.49^a\pm0.04\) | \(0.47^a\pm0.04\) | \(0.46^a\pm0.03\) | \(0.47^a\pm0.00\) |
| \(18:3n-3\) | Linolenic acid | \(1.02^a\pm0.10\) | \(0.99^a\pm0.03\) | \(0.76^b\pm0.03\) | \(1.04^a\pm0.05\) |
| \(22:0\) | Behenic acid | \(0.15^a\pm0.05\) | \(0.09^b\pm0.01\) | \(0.22^a\pm0.00\) | \(0.17^a\pm0.04\) |
| \(\Sigma\) SFA | Saturated | \(36.57 ^a\pm1.02\) | \(35.41^a\pm0.03\) | \(36.62^a\pm0.06\) | \(35.53^a\pm0.03\) |
| Fatty acids | |||||
| \(\Sigma\) MUFA | Monounsaturated | \(55.63^a\pm1.03\) | \(55.80^a\pm0.11\) | \(54.95^a\pm0.11\) | \(55.22^a\pm0.08\) |
| Fatty acids | |||||
| \(\Sigma\) PUFA | Polyunsaturated | \(7.81^c\pm0.01\) | \(8.78^a\pm0.14\) | \(8.43^b\pm0.05\) | \(8.86^c\pm0.40\) |
| Fatty acids |
*Results expressed as mean \(\pm\) standard deviation (SD) of triplicate. Values with different letters on the same line are significantly different (\(p < 0.05\)) by Tukey’s test. ND – Not determined.
The samples showed high values for saturated (SFA) and monounsaturated fatty acids (MUFA) about 35% and 55% respectively. According to Iha et al., 2014, andiroba oil is a rich source of fatty acids such as oleic, palmitic, among others, corroborating the results found here. Bataglion et al. (2014), carried out a chemical characterization using gas chromatography coupled with mass spectrometry (GC-MS), in oils from the Amazon region such as coconut, castor bean and andiroba, acquired commercially. The analysis revealed palmitic, oleic, and stearic acids as the most abundant components, as well as those found in this study.
Regarding the AO and CO samples, there is a higher value in the level of polyunsaturated fatty acids in the commercial oil (CO). There is a strong relationship between the fatty acid profile and lipid oxidation, and the greater the amount of polyunsaturated fatty acids, the greater the possibility of oxidative degradation (Silva et al., 2010). Thus, it appears that the artisanal oil has a greater stability than the commercial oil, which can be explained by the presence of some phenolic substances in the AO, which act as stabilizers (Van, 2010).
However, there was no great variation in the fatty acid profile of the analyzed oils, demonstrating that the commercial oil extraction process does not change the fatty acid composition, a primordial characteristic for the commercialization of this product.
Regarding the microcapsules, the chromatographic analysis showed that the microencapsulation did not interfere in the fatty acid profile of the oils evaluated, see Table 1.
Triacylglycerols identification
Table 2 shows the 13 mass/charge ratios (\(m/z\)) with their possible TAGs, which were determined in the region from (\(m/z\)) 824 to 904. The LAMES Platform was developed for the random configuration of TAGs for vegetable oils (Antoniosi et al., ).
| Molecular | Shortband | \(\mathbf{{m/z}}\) | TAG | TAG estimate (%) | |||
| formula\(^*\) | assigment\(^{**}\) | AO | CO | MAO | MCO | ||
| C\(_{51}\)H\(_{98}\)O\(_{6}\) | \(48:0\) | \(824\) | PPP | \(2.815\) | \(2.287\) | \(2.640\) | \(2.209\) |
| C\(_{53}\)H\(_{98}\)O\(_{6}\) | \(50:2\) | \(842\) | PLP | \(1.980\) | \(1.960\) | \(2.163\) | \(1.917\) |
| C\(_{53}\)H\(_{100}\)O\(_{6}\) | \(50:1\) | \(850\) | POP | \(15.638\) | \(13.771\) | \(14.857\) | \(13.275\) |
| C\(_{53}\)H\(_{102}\)O\(_{6}\) | \(50:0\) | \(852\) | SPP | \(2.068\) | \(1.860\) | \(2.078\) | \(1.917\) |
| C\(_{55}\)H\(_{100}\)O\(_{6}\) | \(52:3\) | \(874\) | PLO | \(7.334\) | \(7.869\) | \(8.114\) | \(7.683\) |
| C\(_{55}\)H\(_{102}\)O\(_{6}\) | \(52:2\) | \(876\) | POO | \(28.958\) | \(27.643\) | \(27.871\) | \(26.599\) |
| C\(_{55}\)H\(_{104}\)O\(_{6}\) | \(52:1\) | \(878\) | SOP | \(7.657\) | \(7.493\) | \(7.797\) | \(7.683\) |
| C\(_{57}\)H\(_{86}\)O\(_{6}\) | \(54:1\) | \(884\) | SOS | \(0.812\) | \(0.900\) | \(0.869\) | \(1.112\) |
| C\(_{57}\)H\(_{100}\)O\(_{6}\) | \(54:5\) | \(898\) | OLL | \(0.745\) | \(1.124\) | \({0.941}\) | \(1.112\) |
| C\(_{57}\)H\(_{102}\)O\(_{6}\) | \(54:4\) | \(900\) | OLO | \(6.790\) | \(7.898\) | \(7.610\) | \(7.697\) |
| C\(_{57}\)H\(_{104}\)O\(_{6}\) | \(54:3\) | \(902\) | OOO | \(17.874\) | \(18.496\) | \(17.427\) | \(17.765\) |
| C\(_{57}\)H\(_{104}\)O\(_{6}\) | \(54:3\) | \(902\) | SLO | \(1.796\) | \(2.133\) | \(2.129\) | \(2.223\) |
| C\(_{57}\)H\(_{106}\)O\(_{6}\) | \(54:2\) | \(904\) | SOO | \(7.090\) | \(7.49\)3 | \(7.314\) | \(7.697\) |
* C: carbon; H: hydrogen; O: oxygen
xxxxxx** Acronyms of TAG - P: palmitic acid (16:0); S: stearic acid (18:0); O: oleic acid (18:1n-9);
xxxxxxxxxL: linoleic acid (18:2n-6)
The highest estimates of triacylglycerols were presented with the amount of carbonation in 52:2, corresponding to 28.958% in AO and 27.643% in CO, followed by 54:3, probable triolein (OOO) being 17.874% in AO and 18.496% in CO. Oleic acid (O, 18:1n-9) was present in all the most abundant TAGs, followed by palmitic (P, 16:0) and linoleic (L, 18:2n-6) acids, consistent with the data shown in Table 1, which also shows these fatty acids as the most abundant.
These data corroborate those found by Cabral et al., 2013 and Bataglion et al., 2014, who used the technique of electrospray ionization mass spectrometry (ESI-MS) to determine triacylglycerols in andiroba oil. Cabral et al., 2013 reported that andiroba oil is mainly composed of TAGs with high levels of oleic, palmitic, stearic, and linoleic fatty acids, as observed in this study.
The purity of vegetable oils is based on measuring the composition of free fatty acids, as well as mono, di and triacylglycerols. In Brazil, it is common to find andiroba oil mixed with cheaper soybean oil (Cabral et al., 2013). Thus, as seen in Table 2, both oil samples had the same lipid profile, demonstrating that the commercial oil used was not adulterated.
Determination of encapsulation efficiency and loading capacity xxxxxxxxxxxxx
The microcapsules had high moisture (> 80%), Table 3, characteristic of a product obtained by complex coacervation, with no significant difference between samples.
| Sample | Moisture (%) | LC (%) | EE (%) |
|---|---|---|---|
| MAO | \(82.83^a\pm0.25\) | \(25.14^b\pm0.90\) | \(70.14^b\pm0.25\) |
| MCO | \(83.10^a\pm0.89\) | \(35.54^a\pm4.24\) | \(99.15^a\pm11.8\) |
*Results expressed as mean \(\pm\) standard deviation (SD) of duplicates.
Values with different letters in the same column are significantly different \((p < 0.05)\).
The highest values of encapsulation efficiency and loading capacity were observed in the MCO sample. A possible explanation for this difference is that in the extraction process several compounds can be extracted with the oil, which could affect the result of the encapsulation efficiency of the artisanal oil. In the process of obtaining commercial oil, there is a centrifugation step after extraction, which could eliminate several compounds, thus increasing the encapsulation efficiency. The encapsulation efficiency is also directly related to the emulsification of the solution (Prata & Grosso, 2015). Commercial oil, by going through the centrifugation step, may be free of some residues, which could interfere with the emulsification of the solution, and consequently, with the efficiency. Therefore, further studies should be carried out to evaluate the composition of these oil samples.
Loading capacity is an important parameter, as it allows estimating the amount of microcapsules to be added to a formulation, to obtain the desired effect of the active ingredient in the final product. Loading capacity results (> 20%) demonstrated the feasibility of applying microcapsules in the preparation of emulsion formulations, with anti-repellent action. It is noteworthy that a low loading capacity can have a high cost, as a larger amount of microcapsule will be needed to reach a desired concentration of the active compound. On the other hand, high loading capacity (> 50%) has the disadvantage of reduced protection of the microencapsulated ingredient. As the concentration of the core increases in the microcapsule, the amount of wall material decreases. Furthermore, the active ingredient can be positioned too close to the microcapsule wall, resulting in a faster release (Shaddel et al., 2018).
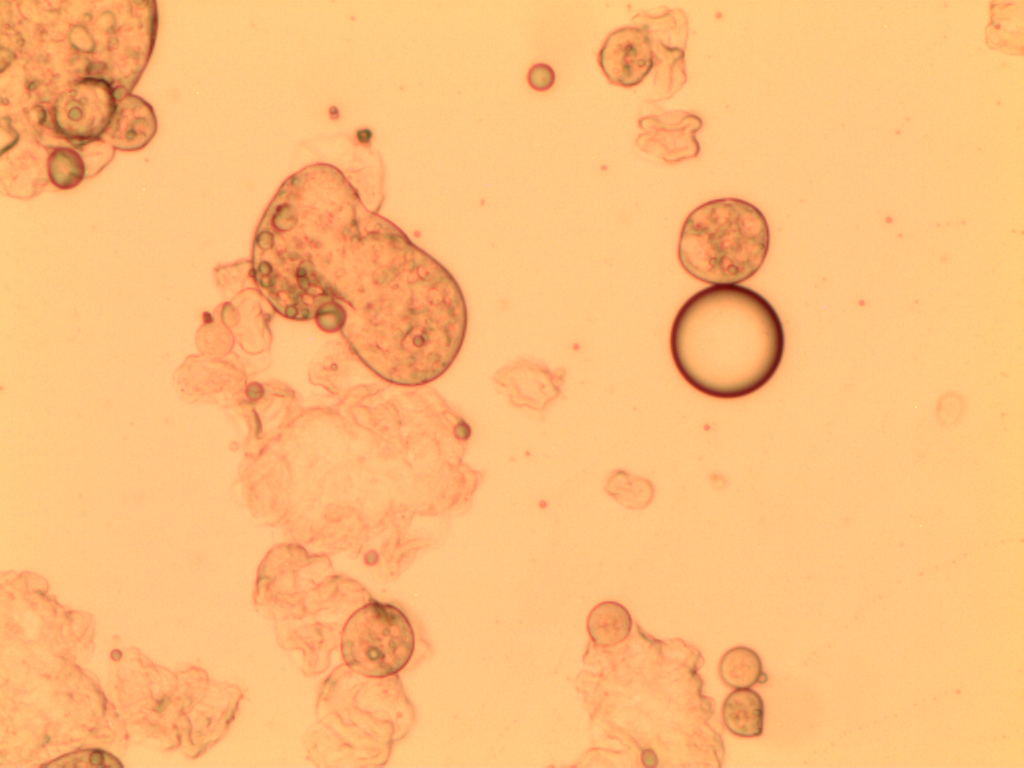
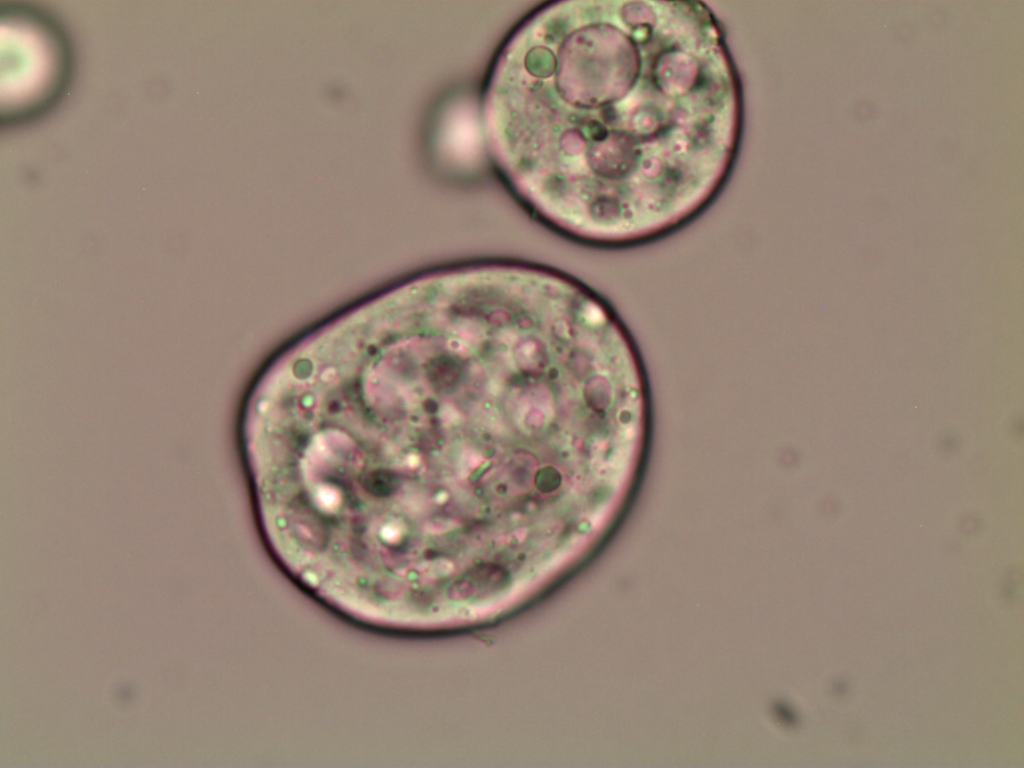
Optical microscopy
The andiroba oil microcapsules (MAO and MCO) had a spherical and oval shape, Figure 1. The MCO sample had a more regular shape when compared to the MAO sample, which may be evidence that some substances present in the artisanal oil may affected the microcapsule formation.
| (a) | (b) |
 |  |
| (c) | (d) |
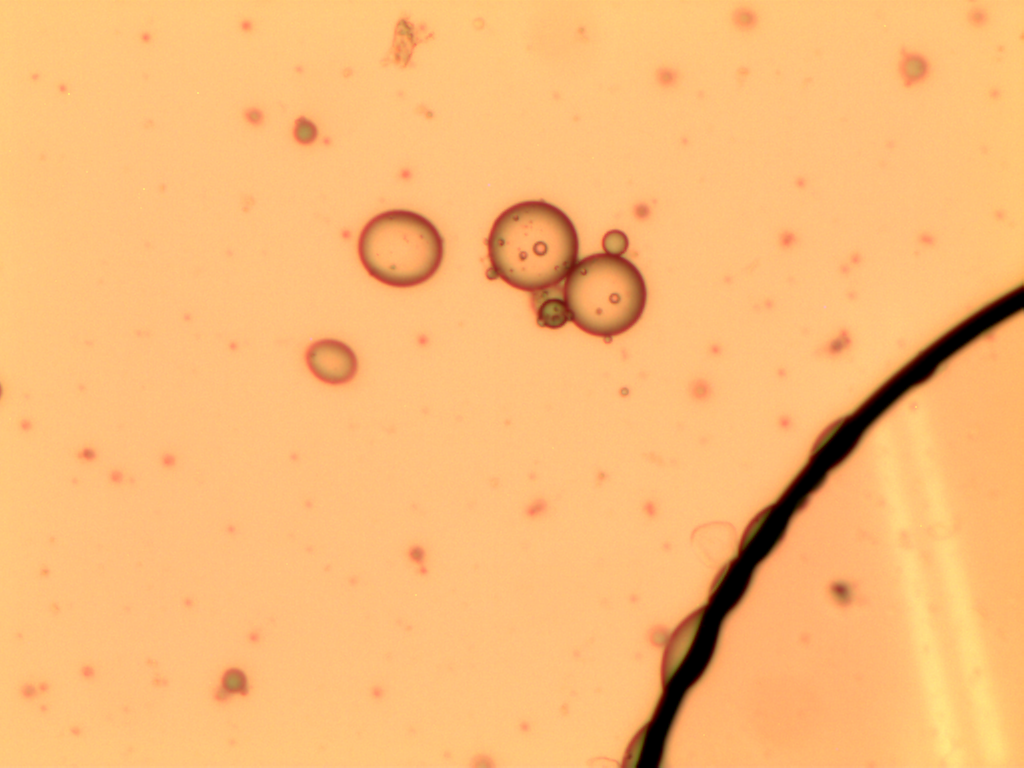 | 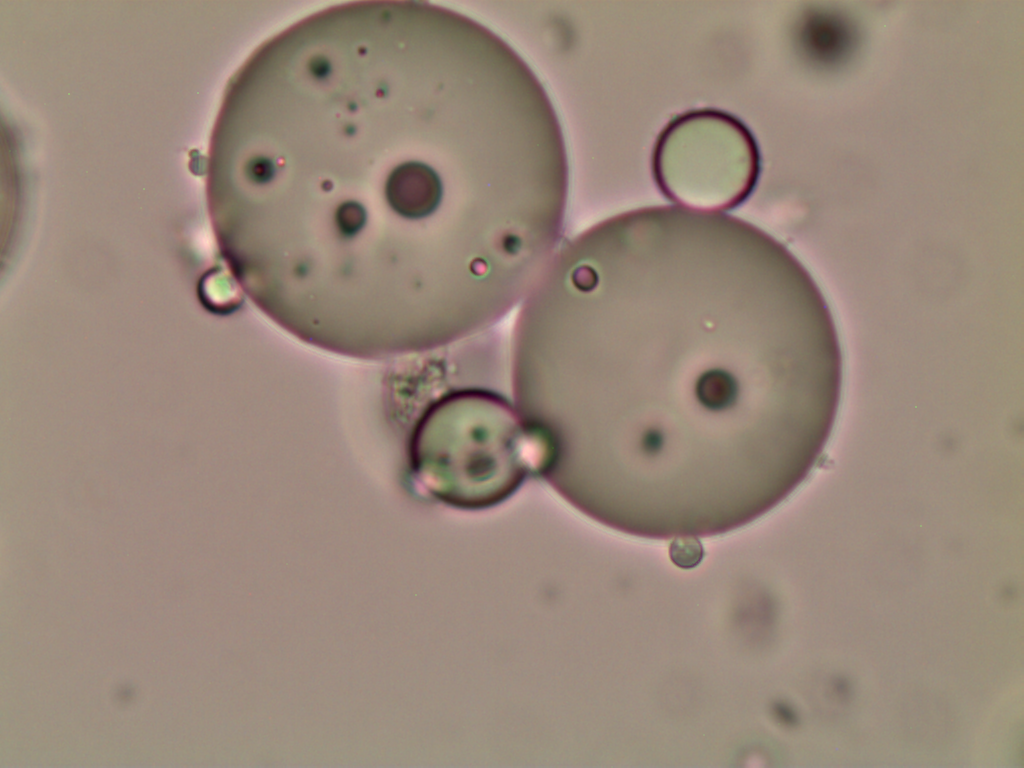 |
It is also observed that the microcapsules are multinucleate, that is, there are several oil droplets trapped in a microcapsule, and with defined walls, which can provide greater protection to the nucleus. Generally, microcapsules obtained by complex coacervation present this multinucleate characteristic, with droplets concentrated in the center, surrounded by layers of encapsulating agents, making them less porous and more robust, and with excellent controlled release characteristics (Yakindra Prasad Timilsena et al., 2017). The MCO sample has only a few scattered spots within the microcapsule, Figures 1(c) and 1(d), indicating the presence of microencapsulated oil. The MAO sample has several dots dispersed within the microcapsule, Figures 1(a) and 1(b), indicating that there are several microencapsulated substances, not just andiroba oil. This result corroborates the encapsulation efficiency and loading capacity, which presented higher values for the MCO sample.
This morphology was also observed by Alvim & Grosso, 2010, who worked with paprika oleoresin microcapsules, in gelatin and gum arabic cross-linked with glutaraldehyde or transglutaminase, and by Comunian et al., 2013, who encapsulated ascorbic acid using gelatin and gum arabic as the wall material.
Scanning Electron Microscopy (SEM)
The micrograph of MAO and MCO indicates a high agglomeration of microcapsules, connected by solid bridges, as also observed in the work by Comunian et al., 2013. However, some spherical shapes are found in the cluster (indicated by red arrows), Figures 2 and 3.
| (a) | (b) |
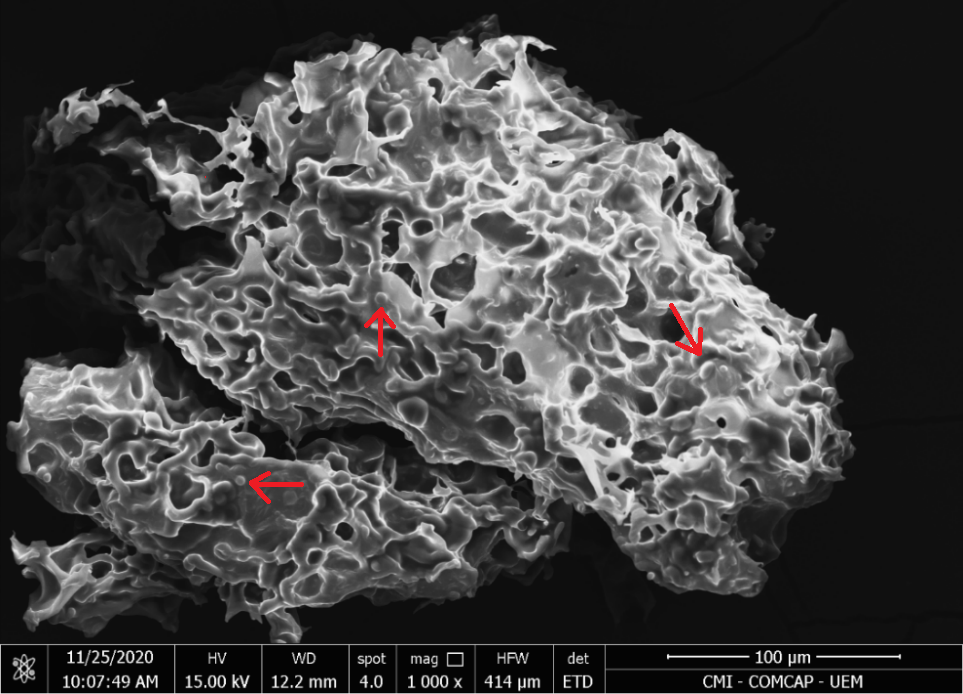 |  |
| (a) | (b) |
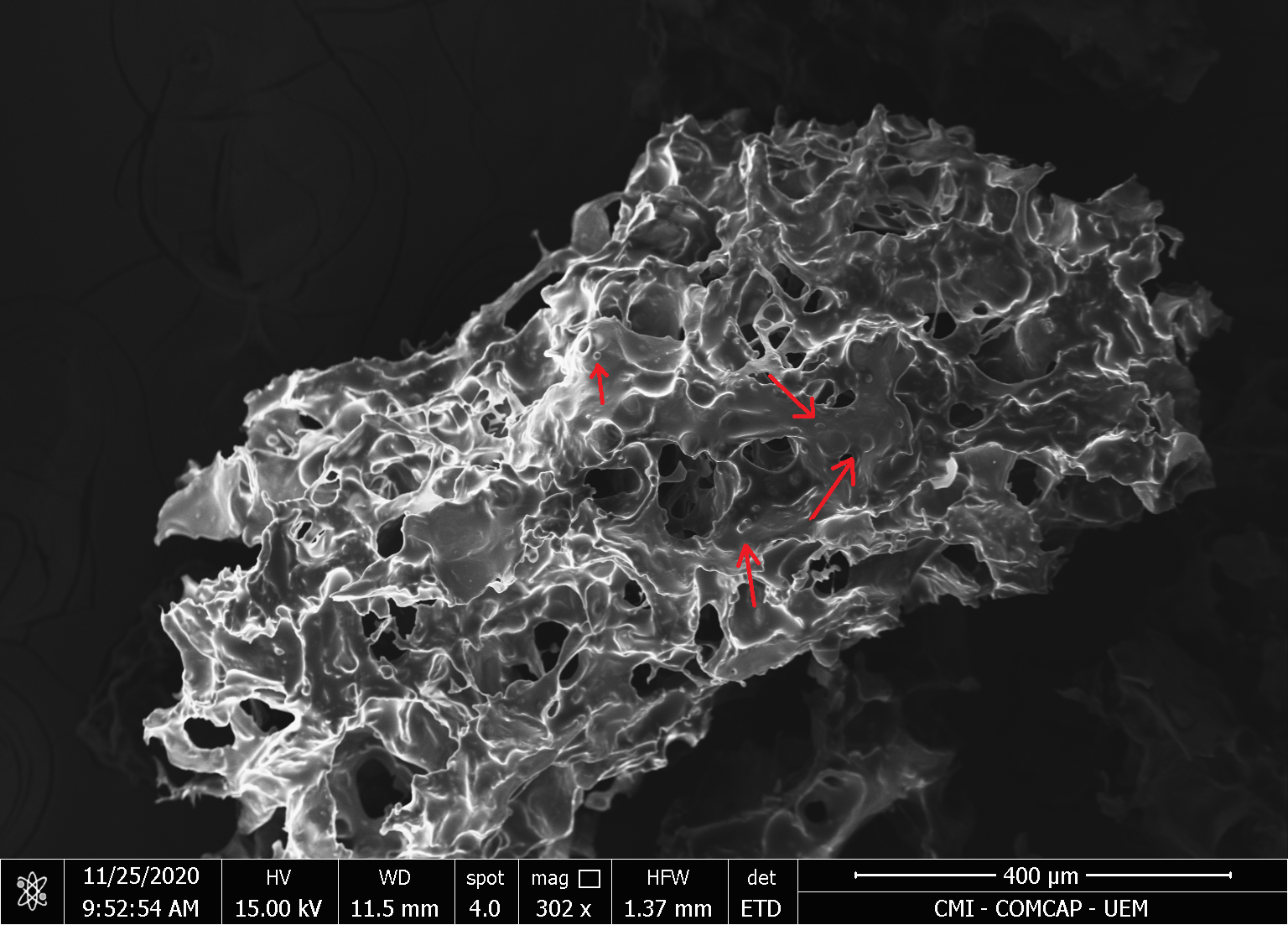 | 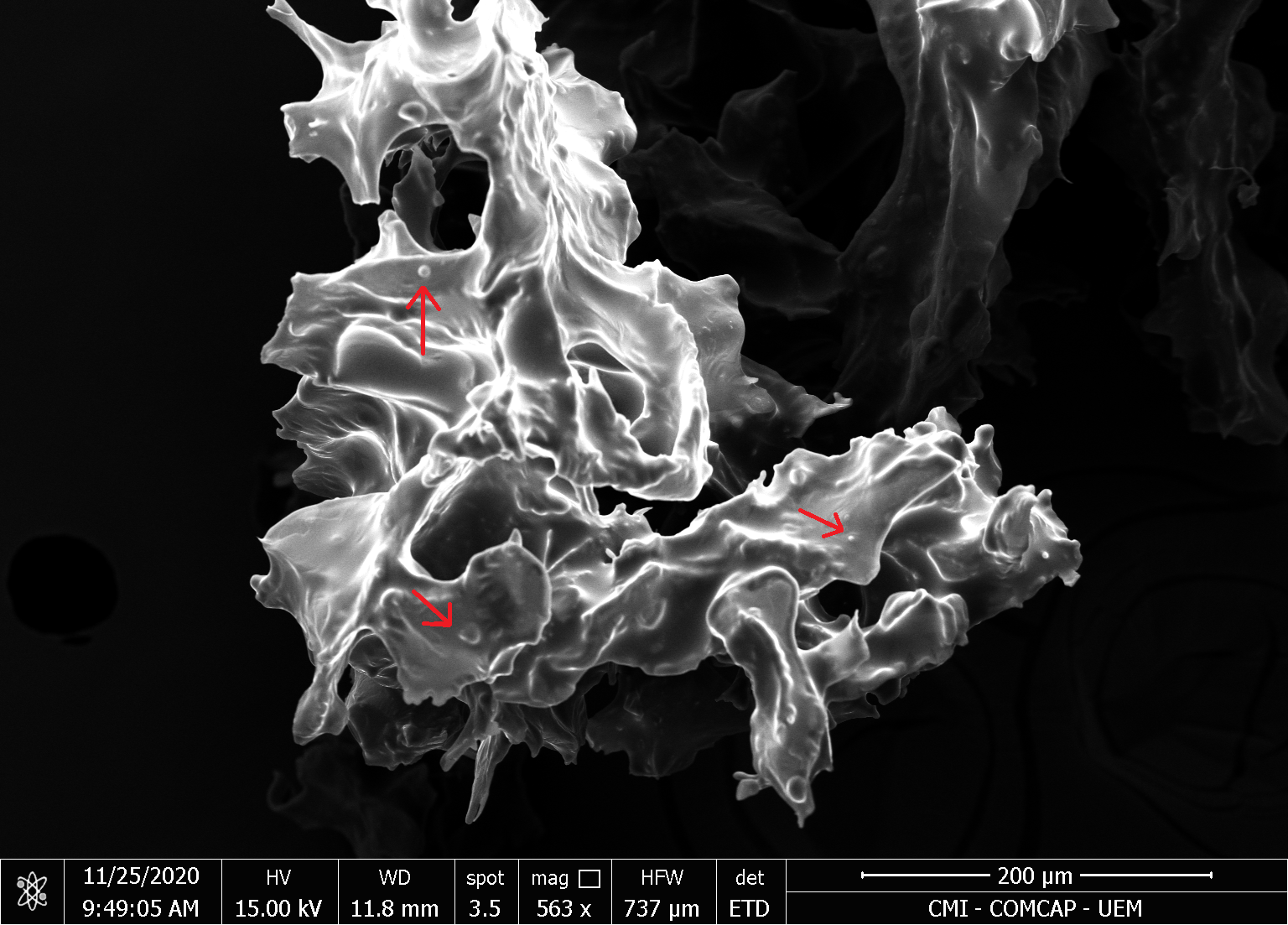 |
Devi & Kakati, 2013 reported that the non-agglomeration of coacervates, produced with gelatin and sodium alginate, would only be possible with the use of a crosslinking agent. Here we chose not to use crosslinking agents, considering that most available agents are not allowed for food applications (example glutaraldehyde), while others are too costly (example transglutaminase enzyme). However, further studies should be carried out with possible crosslinking agents permitted for food applications, as these agents offer incomparable ways to improve the thermal and mechanical properties of complex coacervates during drying and storage, increasing the stability and sustained release of bioactive compounds (Muhoza et al., 2023).
Average diameter and size distribution
The type of oil used in the formulations affected the size distribution of the microcapsules, Figure 4. The MAO sample presented a bimodal distribution, with a peak of greater intensity at 2.33 \(\mu\)m (90%), and a smaller peak at 0.18 \(\mu\)m (10%). The MCO sample, on the other hand, presented a narrower, monomodal distribution, with a single intensity peak at 3.33 \(\mu\)m. According to (Papini et al., 2005), non-spherical particles are measured in all orientations, resulting in a widening of the particle size distribution. This fact corroborates the optical microscopy analysis, Figure 2, where the MAO sample presents some non-spherical microcapsule shapes.
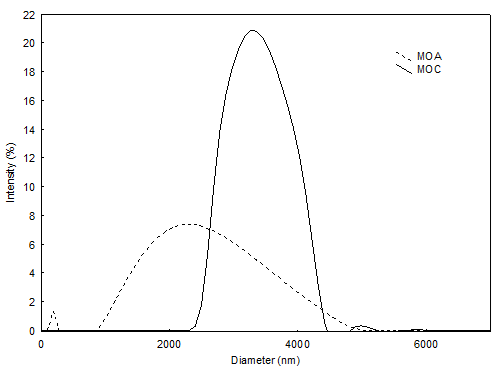
Polydispersity (span) also reflects a larger distribution of microcapsule size, with a higher value for the MAO sample, when compared to the MCO sample, Table 4. A similar result was obtained by Senhorini et al., 2012 with microcapsules of andiroba oil prepared by simple emulsion, followed by evaporation of the organic solvent (Span 1.2).
The span index is often used to express the pattern of particle size distribution. A low Span value reflects a more homogeneous size distribution, while a high value represents a highly polydisperse sample with large variation in particle size (Ilić et al., 2009).
The different compounds present in artisanal andiroba oil may have impaired the oil and matrix interaction, resulting in a greater distribution of sizes. However, the polydispersity value of the MCO sample (0.35) is promising for application of microcapsules in emulsion formulations.
| Sample | Mean Diameter (\(\mu\)m) | \(\mathbf{D_{10}}\) (\(\mu\)m) | \(\mathbf{D_{50}}\) (\(\mu\)m) | \(\mathbf{D_{90}}\) (\(\mu\)m) | Span |
|---|---|---|---|---|---|
| MAO | \(2.33\pm0.83\) (peak 1) | 0.25 | 2.02 | 3.31 | 1.51 |
| \(0.18\pm0.03\) (peak 2) | |||||
| MCO | \(3.33\pm0.41\) | \(2.65\) | \(3.17\) | \(3.78\) | \(0.35\) |
The MAO and MCO samples had little variation in the mean diameter and regular characteristics of microcapsules obtained by the complex coacervation technique, an interesting factor, as it allows a more controlled release of the bioactive compound (Nezamdoost-Sani et al., 2024). In addition, the microcapsules obtained in this study presented a diameter approximately 10 times smaller than the value found by Senhorini et al., 2012, when encapsulating andiroba oil by the simple emulsion technique, followed by evaporation of the organic solvent. In general, smaller particle diameters may represent a kinetically stable emulsion, which may favor the permanence of the bioactive compound in the microcapsules, during the formation of the coacervate (Prata & Grosso, 2015).
Conclusions
In this work, chromatographic analysis of the lipid profile of andiroba oil, obtained by artisanal and commercial processes, was performed, and no great variation was presented in the fatty acid profile of the analyzed oils, demonstrating that the commercial oil extraction process does not change the fatty acid composition, a primordial characteristic for the commercialization of this product. The microencapsulation of andiroba oil was successfully performed, with no negative effect on the lipid profile of the oils evaluated. However, differences were observed in microcapsule characteristics prepared with artisanal and commercial oil, evidencing that the origin of the oil interferes with the formation of microcapsules by the complex coacervation technique. Commercial andiroba oil microcapsules proved to be more spherical, with better homogeneity, greater encapsulation efficiency and loading capacity than artisanal andiroba oil microcapsules, which may favor greater stability and controlled release of the bioactive compound. Further studies should be conducted to evaluate the thermal and oxidative stability of microencapsulated oils, and to determine the potential application of microcapsules as ingredients in the formulation of emulsions, such as cosmetics and repellents.
Author contributions
J.C.M. da Costa: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft. E. da S. Alves: Formal analysis, Investigation. J.V. Visentainer: Methodology, Project administration. A.B.D. Mendes: Methodology, Project administration. M.R. da S. Scapim: Conceptualization, Funding acquisition, Project administration, Writing – review and editing. R. de C. Bergamasco: Conceptualization, Methodology, Project administration, Writing – review and editing.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) for financial assistance.
References
Association of Official Analytical Chemists (2005). Official Methods of Analysis of the Association of Official Analytical Chemists (18th ed.). Gaithersburg.
Alvim, I. D. & Grosso, C. R. F. (2010). Microparticles obtained by complex coacervation: influence of the type of reticulation and the drying process on the release of the core material. 30(4), 1069--1076. https://doi.org/10.1590/S0101-20612010000400036
Ambrozin, A. R., Leite, A. C., Bueno, F. C., Vieira, P. C., Fernandes, J. B., Bueno, O. C., Silva, M. F. G. F., Pagnocca, F. C., Hebling, M. J. A. & Bacci, M. Jr. (2006). Limonoids from andiroba oil and Cedrela fissilis and their insecticidal activity. 17(3), 542--547. https://doi.org/10.1590/S0103-50532006000300017
Amoroso, L., Muratore, G., Ortenzi, M. A., Gazzotti, S., Limbo, S. & Piergiovanni, L. (2020). Fast production of cellulose nanocrystals by hydrolytic-oxidative microwave-assisted treatment. 12(1), 68. https://doi.org/10.3390/polym12010068
Araújo, J. S. F., de Souza, E. L., Oliveira, J. R., Gomes, A. C. A., Kotzebue, L. R. V., Agostini, D. L. S., Oliveira, D. L. V., Mazzetto, S. E., da Silva, A. L. & Cavalcanti, M. T. (2020). Microencapsulation of sweet orange essential oilCitrus aurantium var. dulcis) by liophylization using maltodextrin and maltodextrin/gelatin mixtures: Preparation, characterization, antimicrobial and antioxidant activities. 991--999. https://doi.org/10.1016/j.ijbiomac.2019.09.160
Association of Official Analytical Chemists (2005). Official Methods of analysis of the Association of Official Analytical Chemists. AOAC Intl, Gaithersburg.
Bataglion, G. A., da Silva, F. M., Santos, J. M., dos Santos, F. N., Barcia, M. T., de Lourenço, C. C., Salvador, M. J., Godoy, H. T., Eberlin, M. N. & Koolen, H. F. (2014). Comprehensive characterization of lipids from Amazonian vegetable oils by mass spectrometry techniques. 472--481. https://doi.org/10.1016/j.foodres.2014.07.011
Bligh, E. G. & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. 911--917.
Brito, A. D., Silva, T. F. A., Coelho, R. F. R. & Rosal, L. F. (2020). Saberes e práticas tradicionais da extração do óleo de Carapa guianenses Abul. (andiroba) em área de várzea do município de Igarapé-Mirin, PA. 110--122. https://doi.org/10.33240/rba.v15i3.23165
Cabral, E. C., da Cruz, G. F., Simas, R. C., Sanvido, G. B., Gonçalves, L. D. V., Leal, R. V., da Silva, R. C. F., da Silva, J. C. T., Barata, L. E. S., da Cunha, V. S., França, L. F., Daroda, R. J., de Sá, G. F. & Eberlin, M. N. (2013). Typification and quality control of the andirobaCarapa guianensis) oil via mass spectrometry fingerprinting. 1385--1391. https://doi.org/10.1039/C3AY25743F
Comunian, T. A., Thomazini, M., Alves, A. J. G., de Matos Junior, F. E., de Carvalho Balieiro, J. C. & Favaro-Trindade, C. S. (2013). Microencapsulation of ascorbic acid by complex coacervation: Protection and controlled release. 52(1), 373--379. https://doi.org/10.1016/j.foodres.2013.03.028
Costa-Silva, J. H., Lima, C. R., Silva, E. J. R., Araújo, A. V., Fraga, M. C. C. A., Ribeiro, A. R., Arruda, A. C., Lafayette, S. S. L. & Wanderley, A. G. (2008). Acute and subacute toxicity of thCarapa guianensis Aublet (Meliaceae) seed oil. 116(3), 495--500. https://doi.org/10.1016/j.jep.2007.12.016
Devi, N. & Kakati, D. K. (2013). Smart porous microparticles based on gelatin/sodium alginate polyelectrolyte complex. 117(2), 193--204. https://doi.org/10.1016/j.jfoodeng.2013.02.018
De Kruif, C. G., Weinbreck, F. & de Vries, R. (2004). Complex coacervation of proteins and anionic polysaccharides. 9(5), 340--349.
Fayad, S. J., Ramos, B. G., Soldi, V. & Minatti, E. (2015). Nanopartículas de proteína isolada de soja em água: efeito da força iônica e das concentrações de proteína e surfactante. 91--96. https://doi.org/10.5935/0100-4042.20140295
Antoniosi, N. R., Filho, Mendes, O. L. & Lanças, F. M. (1995). Computer prediction of triacylglycerol composition of vegetable oils by HRGC. 557--562. https://doi.org/10.1007/BF02290268
Hijo, A. A. T., Guinosa, R. E. & Silva, E. K. (2022). Ultrasound emulsification energy strategies impact the encapsulation efficiency of essential oils in colloidal systems. 119179. https://doi.org/10.1016/j.molliq.2022.119179
Iha, O. K., Alves, F. C., Suarez, P. A., Silva, C. R., Meneghetti, M. R. & Meneghetti, S. M. (2014). Potential application of Terminalia catappa L. and Carapa guianensis oils for biofuel production: Physical-chemical properties of neat vegetable oils, their methyl-esters and bio-oils (hydrocarbons). 95--98. https://doi.org/10.1016/j.indcrop.2013.10.001
Ilić, I., Dreu, R., Burjak, M., Homar, M., Kerč, J. & Srčič, S. (2009). Microparticle size control and glimepiride microencapsulation using spray congealing technology. 381(2), 176--183. https://doi.org/10.1016/j.ijpharm.2009.05.011
Marfil, P. H., Vasconcelos, F. H., Pontieri, M. H. & Telis, V. (2016). Development and validation of analytical method for palm oil determination in microcapsules produced by complex coacervation. 94--99. https://doi.org/10.5935/0100-4042.20150164
Matos, E. F., Scopel, B. S. & Dettmer, A. (2018). Citronella essential oil microencapsulation by complex coacervation with leather waste gelatin and sodium alginate. 6(2), 1989--1994. https://doi.org/10.1016/j.jece.2018.03.002
Muhoza, B., Yuyang, H., Uriho, A., Harindintwali, J. D., Liu, Q. & Li, Y. (2023). Spray-and freeze-drying of microcapsules prepared by complex coacervation method: A review. 108650. https://doi.org/10.1016/j.foodhyd.2023.108650
Nezamdoost-Sani, N., Amiri, S. & Khaneghah, A. M. (2024). The application of the coacervation technique for microencapsulation bioactive ingredients: A critical review. 101431. https://doi.org/10.1016/j.jafr.2024.101431
Papini, C. J., Yoshito, W. K., Gouvêa, D. & Neto, R. M. L. (2005). Particle size distribution analysis of an alumina powder: influence of some dispersants, pH and supersonic vibration. 73--78. https://doi.org/10.4028/www.scientific.net/MSF.498-499.73
Pillai, P. & Mandal, A. (2020). A comprehensive micro scale study of poly-ionic liquid for application in enhanced oil recovery: Synthesis, characterization and evaluation of physicochemical properties. 112553. https://doi.org/10.1016/j.molliq.2020.112553
Pizzo, J. S., Galuch, M. B., Santos, P. D., Manin, L. P., Zappielo, C. D., Silva, O. J. Fº., Santos, O. O. & Visentainer, J. V. (2019). Determination of coconut oil adulteration with soybean oil by direct infusion electrospray ionization mass spectrometry. 30(7), 1468--1474. https://doi.org/10.21577/0103-5053.20190042
Prata, A. S. & Grosso, C. R. (2015). Influence of the oil phase on the microencapsulation by complex coacervation. 92(7), 1063--1072. https://doi.org/10.1007/s11746-015-2670-z
Senhorini, G. A., Zawadzki, S. F., Farago, P. V., Zanin, S. M. & Marques, F. A. (2012). Microparticles of poly (hydroxybutyrate-co-hydroxyvalerate) loaded with andiroba oil: Preparation and characterization. 32(5), 1121--1126. https://doi.org/10.1016/j.msec.2012.02.027
Shaddel, R., Hesari, J., Azadmard-Damirchi, S., Hamishehkar, H., Fathi-Achachlouei, B. & Huang, Q. (2018). Use of gelatin and gum Arabic for encapsulation of black raspberry anthocyanins by complex coacervation. 1800--1810. https://doi.org/10.1016/j.ijbiomac.2017.10.044
Shanley, P. & Londres, M. (2011). Andiroba. Fruit trees and useful plants in Amazonian lives. FAO.
Silva, L., Pinto, J., Carrola, J. & Paiva-Martins, F. (2010). Oxidative stability of olive oil after food processing and comparison with other vegetable oils. 121(4), 1177--1187. https://doi.org/10.1016/j.foodchem.2010.02.001
Sousa, R. L., Almeida, B. B., Silva, R. P., Albuquerque, L. C. S. & Cordeiro, Y. E. M. (2019). Óleo de andiroba: extração, comercialização e usos tradicionais na comunidade Mamangal, Igarapé-Miri, Pará. 18(1), 68--81.
Souza, C. R., Lima, R. M. B., Azevedo, C. P. & Rossi, L. M. B. (2006). AndirobaCarapa guianensis Aubl.Embrapa Amazônia Ocidental Documentos, 48, 1-26.
Yakindra Prasad Timilsena, Bo Wang, Raju Adhikari & Benu Adhikari (2017). Advances in microencapsulation of polyunsaturated fatty acids (PUFAs)-rich plant oils using complex coacervation: A review. 369--381. https://doi.org/10.1016/j.foodhyd.2017.03.007
Van Hoed, V. (2010). Phenolic compounds in seed oils. 22(11), 247--249. https://doi.org/10.1002/lite.201000063
Ziming Yang, Zheng Peng, Jihua Li, Sidong Li, Lingxue Kong, Puwang Li & Qinghuang Wang (2014). Development and evaluation of novel flavour microcapsules containing vanilla oil using complex coacervation approach. 272--277. https://doi.org/10.1016/j.foodchem.2013.08.074
International Organization for Standardization (2000). Animal and vegetable fats and oils - Preparation of methyl esters of fatty acids. ISO.
Universidade Federal de Goiás Projetos extras.

