Synthesis and Use of Cellulose/SBA-15 Adsorbent Sheet for Cleaning Polychrome Surfaces on Works of Art
Rizzo, M. M.; Matos, J. R.
DOI 10.5433/1679-0375.2023.v44.47967
Citation Semin., Ciênc. Exatas Tecnol. 2023, v. 44: e47967
Abstract:
Paraloid B72 acrylic varnish has been used in the conservation of cultural properties since 1950’s. With a Tg close to 40 C, some substances can be adsorbed onto varnished surfaces. There are several methods for cleaning works of art, each with its own specificities. This article presents a new alternative method. The cellulose/SBA-15 adsorbent sheets act as containers for the cleaning material, allowing it to act on the surface of the pictorial layer, dissolving and adsorbing unwanted material from the painting, without causing problems such as penetration, paint abrasion, residues, etc. They were tested on original paintings and on a mimetic. The raw materials, artwork, mimetic, and adsorbent sheets, before and after application, were characterized by TG/DTG and DTA; FTIR; XRF; surface digital microscopy and stereoscopy. FTIR characterized the raw materials and adsorbent sheets. The thermal behavior of the raw materials and adsorbent sheets, before and after application, were evaluated by TG/DTG and DTA. The evaluation of the works, the mimetic and the adsorption sheets, before and after application, by XRF showed that there was no damage to the originals and the mimetic. The cellulose sheet/SBA-15 as an adsorbent for B72 in cleaning works of art, using solvent, proved to be effective.
Keywords: artwork restoration, adsorbent, ordered mesoporous silica, cellulose/sbae-15 sheet, conservation science
Introduction
This work presents an adsorption sheet of cellulose and mesoporous silica SBA-15 (Santa Barbara Amorphous-15) for cleaning polychrome surfaces in works of art as an alternative to existing methods. This sheet has been tested to remove B72 varnish (Paraloid B72). B72 acrylic resin is a thermoplastic material that acts as a good adhesive-forming clear, and flexible films and has been used in the conservation and restoration of cultural properties since the 1950s for various purposes, including as a final protective varnish. The B72 resin has a glass transition temperature (\(T_g\)) close to 40 C. With the increase in global temperature in recent years, some substances can become adsorbed on the surface of pictorial layers varnished with this material, making it necessary to remove them. Traditional cleaning (cotton swab and solvents) can cause damage (solvent penetration, abrasion, etc.) to the artwork (L., 2004). Cleaning using aqueous methods (gels and emulsions) can leave residues that interact with the original materials (Wolbers, 1994; Richard, 1990; Wolbers, 2000). The use of rigid gels associated with standard pre-formulated cleaning solutions or emulsions (Bonini et al., 2007) does not solve all cases. Each work of art is unique because of the materials and construction process used by the artist, its history, and the conditions under which it was conserved.
During the creative process, the artist has complete freedom in choosing and using materials. Therefore, the cleaning process of a polychrome surface in a work of art must be studied and defined for each case. The adsorption sheet proposed here must reach the entire surface of the painting layer, accommodating its morphology, dissolving, and removing the protective layer and/or adsorbed pollutants acting as a compress through solvents, solutions, and chelators, among others, without the mechanical process of abrasion and without leaving residues because it is a sheet. The prepared sheets were used on original paintings from different techniques and eras and works made for study, which were varnished with Paraloid B72 to verify its effectiveness. The raw materials, artwork, and adsorbent sheets, before and after application, were characterized using physical-chemical and analytical techniques. This material is unique and innovative. It has not been prepared, tested, or described before and is protected by patent BR 10 2015 013561-0 B1.
Materials and methods
SBA-15 silica, with a highly ordered hexagonal porous structure, due to its large surface area and adequate pore volume, is an excellent material for adsorbing cleaning products, keeping them on the surface and avoiding major complications arising from interaction with the work, such as penetration and retention of these materials, or interactions of any residues with the original.
Materials
For the synthesis of SBA-15: P123 (EO\(_{20}\)-PO\(_{70}\)-EO\(_{20}\)), Triblock Copolymer (Pluronic) [poly(ethylene oxide) – poly(propylene oxide) – poly(ethylene oxide)] (BASF); TEOS (C\(_8\)H\(_{20}\)O\(_4\)Si), Tetraethyl orthosilicate (Aldrich); HCl (Merck); N\(_2\) and synthetic air (Oxilumen); filter paper (Prolab), were used.
To check the adsorption capacity of the silica: 20 and 50 mL glassware with lids (Supplier Pack Rio); solvents: \(n\)-hexane, dimethylformamide (DMF), water, or xylene (Anidrol supplier).
To obtain cellulose fibers: Pinus elliotti dry pulp (Suzano Papel e Celulose Group).
To prepare the adsorption sheets: 20-liter bathtub (Plastitécnica); Mold (form and counter-form); Mechanical mixer (Quimis); Analytical balance (A. Scientific); CHUBBY GA2400 high-frequency generator (Manufacturer Thornton); Ultra Torex Poly Tron PE2007 disperser (Kinematica A.G. Dispense and Mixing Technology); Beakers of various sizes (Anidrol).
To check the behavior of B72: Polyester film (25 microns thick); Paraloid B72\(^{TM}\) Resin, Nikon Microscopic Binocular Magnifier.
To check the effectiveness of the sheet: four original paintings and a collage.
Methods
Synthesis of mesoporous silica SBA-15
The synthesis and characterization of mesoporous silica SBA-15 were performed at the LATIG - Prof. Doctor Ivo Giolito Thermal Analysis Laboratory, according to Matos et al., 2001, with small modifications.
As a general procedure, the surfactant P123 was dispersed in aqueous hydrochloric acid solution deionized water and in hydrochloric acid medium under mechanical agitation until a homogeneous dispersion was obtained. P123 acts as a template or structure guide for obtaining SBA-15. After total dispersion of the P123 copolymer, the silica precursor TEOS was added under stirring for at least 5 minutes. The reaction mixture was then transferred to a Teflon flask, which was hermetically closed, placed in an oven at 100 C, and kept for 48 hours. Under these conditions, the reaction mixture is subjected to hydrothermal treatment under autogenous pressure (self-generated) and then precipitation and polycondensation of the silica in a hexagonal structure templated by the surfactant occurs.
After hydrothermal treatment and cooling, the Teflon flask is opened to remove the reaction product in the form of a gel. This gel is dispersed in deionized water, and the material is subjected to filtration under reduced pressure and thoroughly washed with deionized water.
After filtration, After filtration, the SBA-15 silica as synthesized (SBA-15/as) was dried at 100 C in an oven. Then, the SBA-15/as is subjected to calcination under a dynamic N\(_2\) atmosphere up to 540 C for 2 hours. At this temperature, the atmosphere is switched to synthetic air and maintained at 540 C for about 6 hours to complete the calcination process. Calcined Silica SBA-15 is a very light, fine, white powder. This is the material to be mixed with the cellulose pulp to obtain the sheets.
Nitrogen adsorption/desorption measurements were performed using a Micromeritics adsorption analyzer (model ASAP 2020). The calcined SBA-15 sample was degassed under reduced pressure at a temperature of 200 C before measurement.
The isotherm was recorded at 77K over a relative pressure range of 10\(^{-6}\) to 0.995 using N\(_2\) with 99.998% purity. The specific surface area was evaluated by using the Brunauer–Emmett–Teller (BET) method (J. Rouquerol et al., 1994) from the desorption data in the relative pressure range (P/P\(_0\)) from 0.04 to 0.20. The pore volume was calculated from the amount adsorbed at a relative pressure of 0.99. The pore size distribution was calculated using the Barrett-Joyner-Halenda (BJH) method (Barrett et al., 1951) from the desorption curve data.
Verification of the adsorption capacity of silica in different solvents
To verify the adsorption capacity of calcined SBA-15 in relation to different solvents, the following methodologies were used: system with thermal treatment (with TT) and system without thermal treatment (without TT).
In the system without TT, SBA-15 was placed inside open glasses, which in turn were placed inside other larger glasses with lids, each containing one of the following solvents: n-hexane, dimethylformamide (DMF), water, or xylene at a level below the height of the glass containing silica. Each set was closed and kept at rest for 24 h. All components were weighed, and the silica from each experiment, after adsorption, was subjected to thermogravimetric measurements.
In the TT system, the silica was kept in an oven at 100 C for 90 minutes to eliminate physisorbed water or any other volatile substance from the surface of the silica. After this treatment, the opened flasks were kept under saturated atmospheres of the same solvents described above for 48 h. In this case, the materials after adsorption were also subjected to thermogravimetric measurements.
Formation of adsorption sheets with cellulosic fibers and SBA-15 silica
The adsorption sheets for removing the B72 varnish applied on works of art were obtained using the methodology used in the manufacture of handmade paper (Asunción, 2002).
The previously weighed cellulose fibers were submerged in 1.5 L of deionized water for 24 h for hydration. The wet paste (water + fibers) was mechanically agitated for 15 minutes. While stirring, the adhesive was added. The entire mixture (water + fibers + adhesive) was placed in a bathtub, and deionized water was added until a volume of 15 L was reached. The suspension was manually stirred with the aid of a wooden spatula.
In parallel, the previously weighed dry SBA-15 silica was placed in a 25-mL container containing deionized water. The mixture (silica + water) was placed in the high-frequency generator device and then in the Ultra Torex device for total disaggregation of the particles. This mixture was added to the bathtub containing the fibers and adhesive. The suspension was again stirred manually using a wooden spatula.
For the formation of sheets, the material in aqueous dispersion was collected with a mold for handmade paper composed of two parts: form and counter-form.
Several concentrations of fibers in water were used to form the sheets, which resulted in sheets with different weights; however, the best result was obtained with the one containing 1 % of fibers in water. Similarly, the ratio of adhesive to collage was varied. The one that presented the best result had 2% of adhesive in water. The concentration of fibers in water to form the film and the height of the counter form determine its thickness and grammage after drying, just as the concentration of adhesive in the paste determines its permeability and water resistance.
The proportion of silica in relation to the number of cellulose fibers also varied. Tests were performed with 0, 1, 2, 5, 10, 20, 30 and 50%. The best result was obtained for the material with 50% of the mass of the fibers in the paste. The silica-free film was used for reference, comparison of results, and verification of the effectiveness of the method.
Characterization of samples by thermal analysis (TA)
The thermogravimetry (TG), differential thermal gravimetry (DTG), differential thermal analysis (DTA) curves were obtained using the simultaneous TG/DTA system (named by Shimadzu as DTG-50), under dynamic air atmosphere (50 mL/min), heating rate of 10 C/min, and platinum crucible containing approximately 15 mg of sample.
Characterization by Fourier transform infrared spectroscopy (FTIR)
The absorption spectra in the infrared region of the samples were obtained using Perkin Elmer-Frontier equipment in the region of 4000 to 400 cm\(^{-1}\).
Original paintings and a collage mockup
To prove the effectiveness of adsorption sheets incorporated with SBA-15 silica in removing Paraloid B72\(^{TM}\) varnish on pictorial layers, four original easel paintings from different eras made with different materials and techniques by different artists were used: “Madonna with Child” oil painting on canvas, by unknown painter, 19\(^{th}\) century (MdÓT); “Madonna with Child”, oil painting on metal, by unknown painter, 19\(^{th}\) century (MdÓM); “Psychedelic Fish” (tempera painting on canvas, painter Francisco da Silva, 1973 (PxLT); “Landscape with Beach” (acrylic painting on Eucatex; a high density fiberboard, painter Guiga, 1990 (PrRE). Over each one, a thick layer of Paraloid B72\(^{TM}\) varnish was applied in a stripe shape.
A collage with butterfly wings was mimicked. Four wings of a dead butterfly found in Horto Florestal in São Paulo were glued onto a 9.5 cm \(\times\) 9.5 cm tile. With two facing up and two facing down, we refer to this work as: “Butterfly Wings”, mixed technique with collage, made by author Marcia Rizzo, 2014 (AbGZ). Several layers of Paraloid B72\(^{TM}\) were applied to the set with a low-pressure compressor so as not to damage the work.
Another collage mockup was made for exposure to the environment and verification of the behavior regarding the adsorption of pollutants. A layer of Paraloid B72\(^{TM}\) was applied over a polyester film that was exposed in an open environment, but covered for 4 months, in the summer, in the city of São Paulo/Brazil.
Application of adsorption sheets to remove Paraloid B72\(^{TM}\) varnish from artworks and collages
The behavior of the sheets was tested on original works of art, making the study very reliable.
To test the efficiency of the sheet, the four paintings and the butterfly wing artwork were pre-characterized with X-ray fluorescence using Brucker’s portable TRACER III SD equipment. This procedure was used to ensure that no pigment would be improperly removed from the work with the application of the film. Likewise, the sheets before and after use were characterized.
Then, the reference film without silica, and the sheets with different proportions of silica, were cut into rectangles of approximately 3.0 cm \(\times\) 0.5 cm. These samples were placed dry on on the surface of the pictorial layer with Paraloid B72\(^{TM}\) varnish applied and 4 drops of solvent were deposited on each of them. The solvent chosen was xylene, which solubilizes Paraloid B72\(^{TM}\), and does not affect the artworks neither the collage mockup. On the other hand, ordered mesoporous silica SBA-15 has a high affinity for the adsorption of aromatic hydrocarbons.
Fourier transform infrared spectra of Paraloid B72\(^{TM}\), pure adsorption film and adsorption film used to remove Paraloid B72\(^{TM}\) were also recorded.
Observation under microscopic binocular loupe
The polyester film coated with Paraloid B72\(^{TM}\), which was exposed for 4 months, was observed with a Nikon microscopic binocular loupe, with an increase of 80 \(\times\) to verify the adsorption of particles.
Results and discussion
The characterization of the raw materials, the sheets with and without silica, before and after the application on the artworks and on the collage mockup; and also the artworks and the collage mockup before and after the application of the sheets was carried out.
Thermal analysis (TA)
Figure 1 illustrates the TG/DTG and DTA curves of the pure Paraloid B72\(^{TM}\) sample (B72). The TG/DTG curves show that the sample is thermally stable up to about 238 C and decomposes in two main stages, between 238 and 600 C. The first stage occurs in the range of 238 to 428 C, (\(\Delta m_1 =\) 61.96 % and T\(_{peak~DTG} =\) 329 C). The second stage occurs between 428 and 600 C (\(\Delta m_2 =\) 33.96 % and T\(_{peak~DTG} =\) 370 C) and is due to the completion of thermal decomposition. At a temperature of 600 C, as expected because it is organic matter, the mass loss is complete.
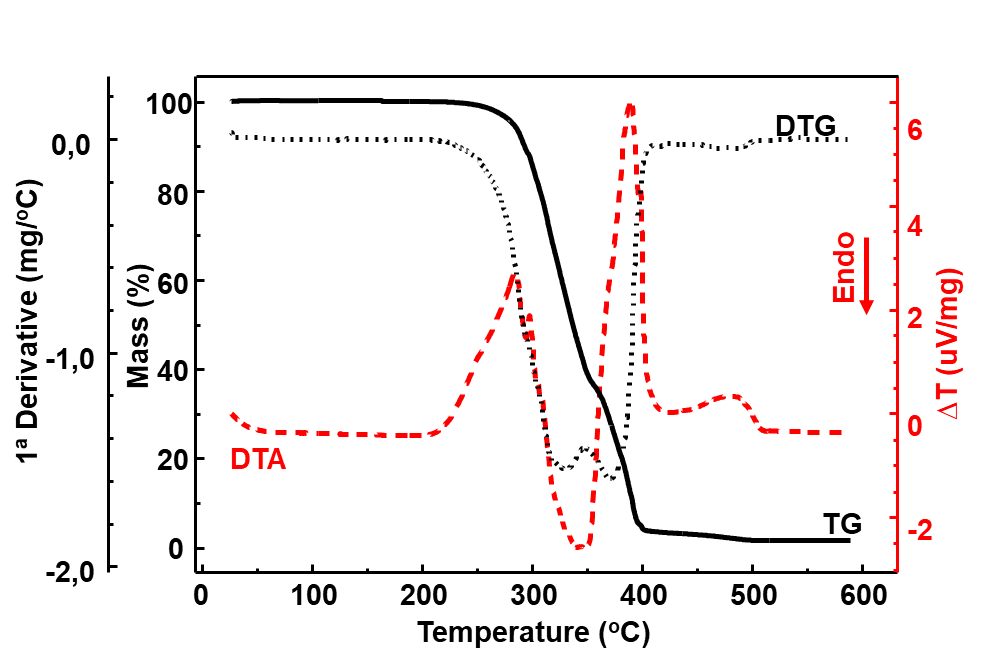
In Figure 1, the DTA curve shows a baseline variation between 25 and 60 C, observed when expanding this temperature range, which is characteristic of the glass transition of the material (\(T_g\)), which appears in the material specification data as 40 C.
To measure the \(T_g\) of the Paraloid used in this study, two subsequent heatings were performed on the sample, as illustrated in Figure 2. The first step was to eliminate the thermal history of the material and the second heating to determine the \(T_g\), according to the recommendations of the standard (American Society for Testing & Materials, 2012) and the literature (Schilling, 1989).
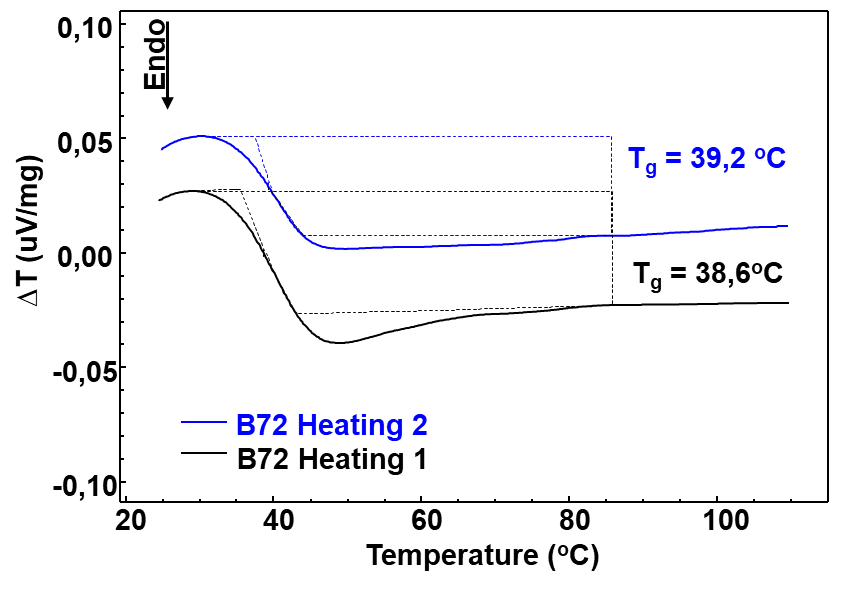
A \(T_g\) value of 39.2 C was found, see Figure 2. This \(T_g\) result shows softening of the material from 39 C onwards, however, the TG/DTG curves show mass loss only above 200 C. The DTA curve did not show a melting peak of the material, but a visual comparison of an isolated sample before and after heating to 300 C confirmed that the material underwent a melting process at the beginning of the thermal decomposition step.
As also observed in the TG/DTG curves, the DTA curve shows that the thermal decomposition process of the sample starts around 238 C and occurs in four main stages. The first step is exothermic and occurs with two superimposed events (T\(_{peak} =\) 248 and 285 C). The second event is endothermic and occurs between 310 and 360 C with T\(_{peak~DTA}\) at 347 C. The third event is exothermic and occurs between 360 C and 416 C, with principal T\(_{peak~DTA}\) at 390 C. The fourth event is also exothermic and occurs between 416 and 520 C, with T\(_{peak~DTA}\) at 483 C.
The softening of the material close to room temperature, especially in Brazil, indicates that Paraloid B72\(^{TM}\) is not suitable for use as a final protective layer on works of art. Figure 3 illustrates a thick layer of Paraloid that was applied over a polyester film and exposed in an open but covered area in São Paulo/Brazil in the south zone for four months.
(a) (b)
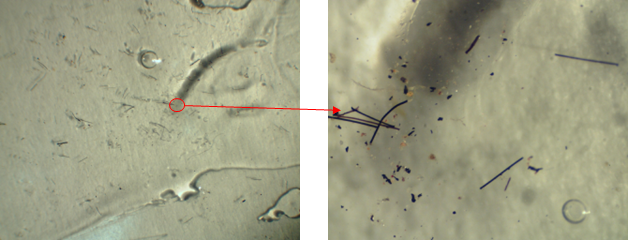
The enlargement of a detail in Figure 3(a) is illustrated in Figure 3(b). The particles suspended in the air, resulting from pollution, adhered to the surface of the Paraloid B72\(^{TM}\) film. As can be inferred, the same occurs with works of art in which Paraloid was used as the final protective varnish. The air we breathe is full of particulate matter of different dimensions, which depending on size and density remain suspended for a longer or shorter time, depending on the atmospheric conditions.
Figure 4 illustrates the overlapping of the TG and DTG curves of the pure SBA-15 silica samples and this silica without thermal treatment, as used in everyday life, but saturated with different solvents for 24 hours [n-hexane (n-H), water (H\(_2\)O), dimethylformamide (DMF), and xylene(X)].
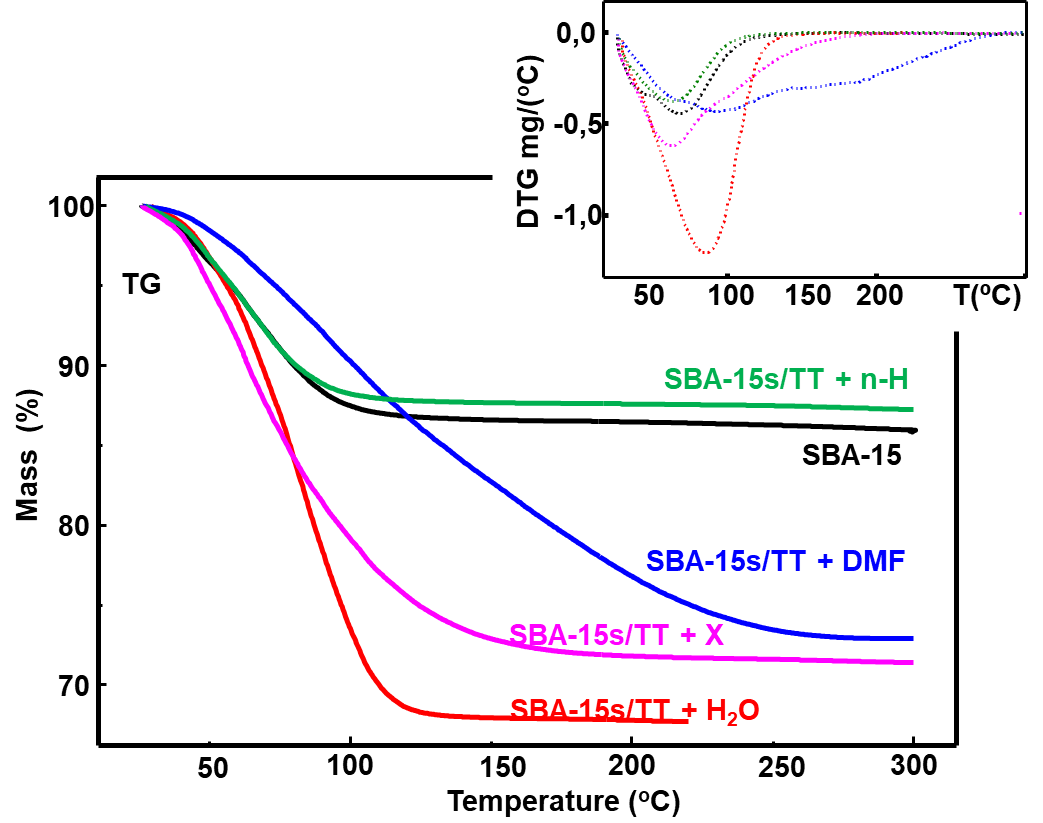
In Figure 4, the TG/DTG curves show that for these samples, there was a greater adsorption of H\(_2\)O, followed by X, DMF, and n-H. This can be seen from the mass losses that occurred, respectively: 32.30%, 28.62%, 27.10%, and 12.75% due to the release of adsorbed solvents. In the case of the n-H sample, the mass loss was smaller than that observed for pure silica. This is because n-H has a high vapor pressure (200 mm Hg at 31.6 C – product specification sheet), i.e, it is a very volatile substance, evaporating completely in the weighing process, even before the sample is placed in the thermobalance sample holder.
Pure silica, in turn, adsorbs about 10% of its mass in ambient water. On the other hand, it was observed that the elimination of DMF from the porous silica matrix occurred more slowly and at higher temperatures compared with other solvents. This evidence serves as an alert to the harmful effects of the use of solvents in the traditional method of cleaning polychrome surfaces such as paints, particularly, dimethylformamide (DMF) and xylene(X).
The polychrome surfaces of easel paintings, especially old ones, behave like porous bodies (L., 2004), and can be compared to silica in this study. In the traditional method of cleaning works of art, which uses a cotton swab moistened with the solvent, the solvent penetrates into the porous pictorial layer, which, as the experiment demonstrates, can be retained in the work due to the difficulty of evaporation.
Some scientists have studied the evaporation and retention of solvents in restoration processes using marked molecules (Baij et al., 2020; Phenix et al., 2020; Masschelein-Kleiner & Deneyer, 1981; Dauchot–Dehon, 1975). Others preferred the gravimetric method using a balance, where tests were performed by deposition of a known amount of solvent on a paint fragment with a standard surface (L., 2004). The results of these measurements indicated that most solvents evaporate in two stages, a fast one, followed by a slower one, where the solvent remains gradually evaporated, in the same way as was observed in the present study with silica, since both the polychrome surfaces of frames and silica are porous bodies.
Among the solvents used in this study to evaluate adsorption on silica, DMF is the most penetrating and is eliminated at a higher temperature. With the use of film, the solvent does not penetrate the porous body of the pictorial layer; it acts only on the surface, dissolving and carrying the varnish that one wants to remove; therefore, among others, there is no problem of
Figure 5 illustrates the overlap of the TG, DTG and DTA curves of the samples, where:
B72 film, black color;
film formed by vegetable cellulose (V) from the Pinus elliotti plant (P), at a concentration of 1% in water (1), bonded with Celvol 107® polyvinyl alcohol adhesive (A), at a concentration of 2% in water (2), with 50% silica in relation to the mass of fibers incorporated in the paste CVP(1)A(2)[SAB15(50)], in blue color;
of the same film after being used to remove the Paraloid B72\(^{™}\) from the MdÓT work, therefore with the impregnated resin, in red color.
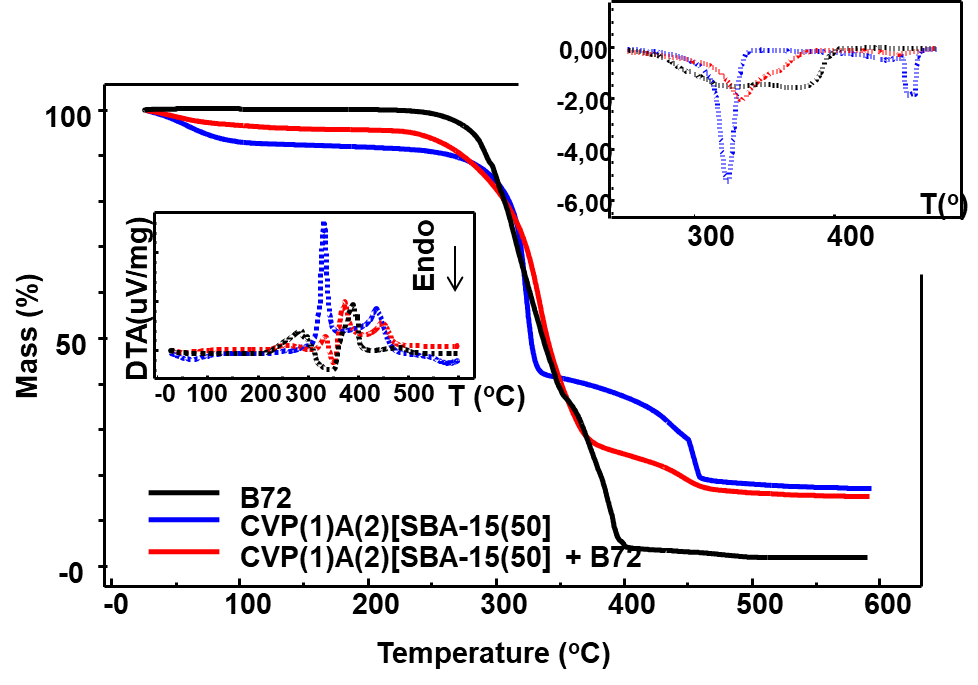
It can be seen from the overlapping of the TG curves in Figure 5 that the film CVP(1)A(2)[SAB15(50)]+B72, comparatively, gathered the thermal characteristics of the other two samples, since it is the impregnation of B72 varnish on CVP(1)A(2)[SAB15(50)]. The TG/DTG curves showed a mass loss of 4.04% between 25 and 150 C, which corresponds to the elimination of physiosorbed water, which is practically half the value of mass loss found for the pure film (7.58%), since the pure Paraloid does not present mass loss in this temperature range.
The film is thermally stable between temperatures of 150 and 262 C and decomposes in two main stages between 262 and 550 ºC. The first step occurs between 262 and 400 C (\(\Delta m_1 =\) 71.24% and T\(_{peak~DTG} =\) 333 C), and the second between 400 and 550 C (\(\Delta m_2 =\) 9.24% and T\(_{peak~DTG} =\) 447 C).
At a temperature of 600 C, the percentage of residues was 15.5%, which corresponds to the silica content in the film. The DTA curve of the CVP(1)A(2)[SAB15(50)]+B72 film corresponds to the sum of the thermal events observed in the DTA curve of the Paraloid sample and the DTA curve of the film without this resin. This result leaves no doubt that the Paraloid was removed from the pictorial layer with the aid of the solvent, and was therefore impregnated in the film. The absence of other thermal events indicates that the solvent has completely evaporated after the dissolution of the Paraloid and its impregnation on the film.
Nitrogen adsorption/desorption isotherm
The nitrogen adsorption/desorption isotherm and the pore size distribution curve of the mesoporous silica sample used in this work, synthesized in LATIG according to the IUPAC classification, have the profile of type IV mesoporous samples, with class 1 hysteresis (H1). Data processing indicated that the sample had a surface area (S\(_{BET}\)) of 821 m\(^2\)·g\(^{-1}\), pore volume (V\(_{PT}\)) of 1.20 cm\(^{3}\)·g\(^{-1}\), and average pore diameter (\(\emptyset_{P}\)) of 10.2 nm. These results are coherent for SBA-15-type silica, which is a good adsorbent, and it is also possible to highlight the narrow pore size distribution, between 9 and 13 nm of this material. These characteristics are similar to those of the SBA-15 sample reported by Matos et al., 2001, but with a higher S\(_{BET}\).
Infrared Spectroscopy (FTIR)
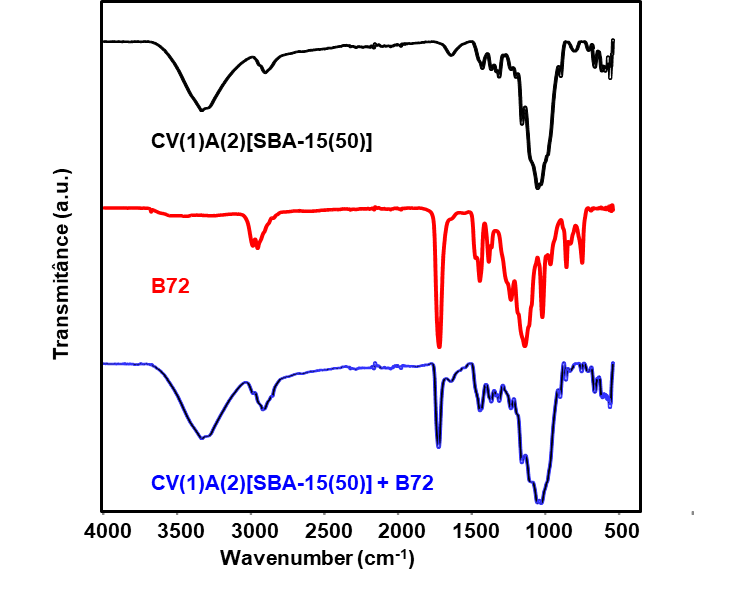
Figure 6 illustrates the overlap of the infrared absorption spectra of the samples:
B72;
CVP(1)A(2)[SBA-15(50)]+B72;
CVP(1)A(2)[SBA-15(50)] for better visualization and comparison. The spectrum of the CVP(1)A(2)[SBA-15(50)] film, blue line, after being used to remove Paraloid B72\(^{TM}\) varnish from the MdÓT work, shows the characteristic absorption bands of the film and the varnish.
The intense peak at 1721 cm\(^{-1}\) (C \(=\) O bond stretching) is characteristic of Paraloid B72\(^{TM}\).
The bands in the region between 3333 and 2917 cm\(^{-1}\) refer to the vibrational stretching modes of the O\(-\)H group of cellulose, which, in addition to the presence of this group in the sample, can refer to the presence of water adsorbed from the environment.
The characteristic absorption bands of SBA-15 silica are also observed, for example at: 1085 cm\(^{-1}\) (asymmetric stretching of the Si-O-Si bond and those referring to CVP samples and those observed between 1480 and 1300 cm\(^{-1}\).
X-ray fluorescence (XRF)
The purpose of carrying out these measurements was to verify that the film only removed B72; that is, no pigment was removed with the varnish. For this purpose, the pigments present in all artworks and the constituent elements of the AbGZ work were characterized. As well as the areas where the cleaning sheets were applied, before and after application.
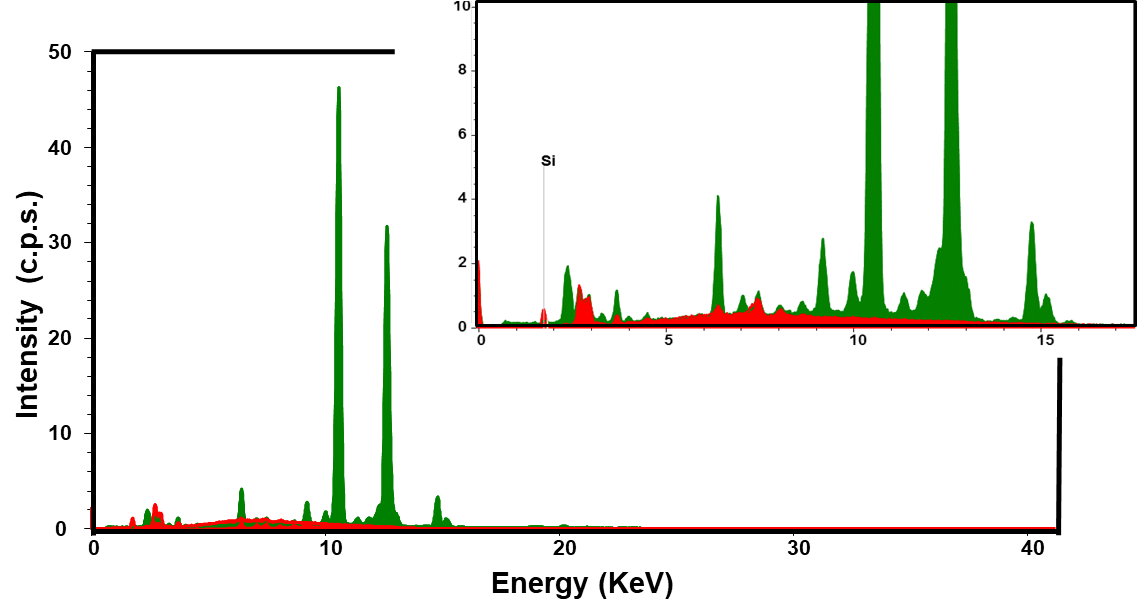
Figure 7 illustrates the superimposition of the sample spectra of the film CVP(1)A(2)[SBA-15(50)] after it was applied to the skin of Baby Jesus in the work MdÓT (skin with pigments containing Ca, Fe, Zn, Hg and Pb) for removal of B72 and the spectrum from the same point before application. The comparison between the spectra leaves no doubt that there was no removal of any inorganic material in the work.
The results of the “blanks” (without sample), made with 15 kV of energy and current of 3 \(\mu\)A, and, 40 kV of energy and current of 20 \(\mu\)A, the following elements were encountered: Ar (from the air), Rh (from the tube X-ray tube), Ni (metal used in the X-ray tube), Sn (from the solder), Cu, Zn, Fe (there are traces of these elements in the Al alloy present in the device), Ti (from the vacuum window), and Pb (from the sample holder).
Organoleptic observation (reflected light)
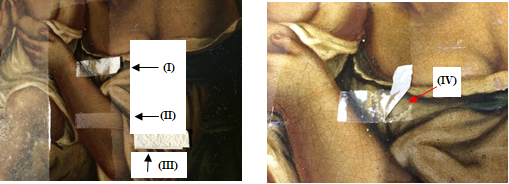
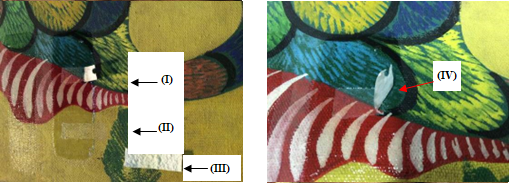
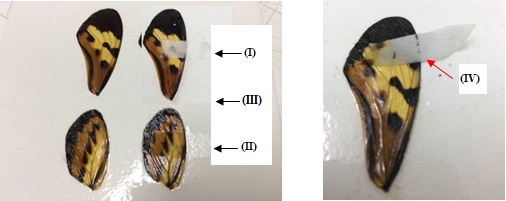
Figures 8 to 11 illustrate the three original paintings and the collage mockup after the application of sheets with and without silica to remove the Paraloid B72\(^{TM}\) varnish.
(a) (b)
(a) (b)
(a) (b)
(a) (b)
| (a) | (b) |
 |  |
| (c) | (d) | (e) |
 |  |  |
| (f) | (g) |
 |  |
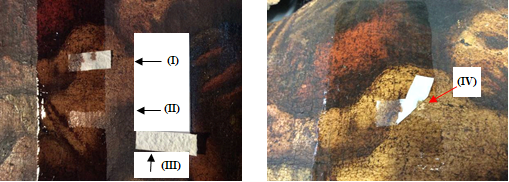
It can be seen in Figures 8(a), 9(a), 10(a), and 11(a) that the film with silica (II) and (III) perfectly removed the varnish without damaging the works, while the film without silica remained adhered to them (I). Attempting to remove them could damage the pictorial layers, as can be seen at point (IV) in Figures 8(b), 9(b), 10(b), and 11(b). The original paintings were not damaged during this experience. The sheet adhered could be removed by solvents.
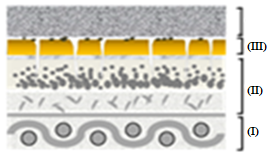
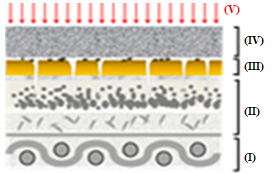
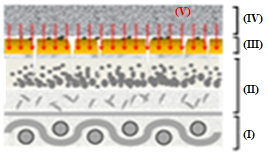
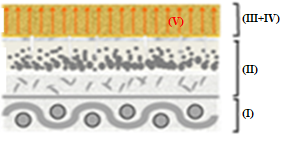
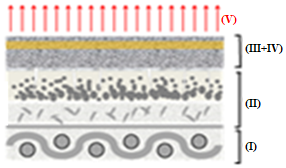
Figure 12 depicts the representative scheme of the action of sheet CVP(1)A(2)[SBA-15(50)] in the removal of B72 from pictorial layers: (I) support, (II) pictorial layer without varnish, (III) B72, (IV) CVP(1)A(2)[SBA-15(50)], and (V) X.
Figures 12(a)-(g) illustrate the action scheme of the cellulose film incorporated with silica in the removal of B72, depicting a process describable in seven steps:
represents the pictorial layer of a painting with Paraloid B72\(^{TM}\) applied;
cellulose film incorporated with silica is placed on the workpiece;
the xylene solvent is dripped onto the film that is already in contact with the work;
the varnish is then solubilized by coming into contact with the film soaked in xylene;
the Paraloid B72 solution with xylene is adsorbed by the silica film due to its excellent adsorbent characteristics, leading to migration from the pictorial layer to the film
impregnation by the solution (varnish + solvent) of the painting, in which the solvent evaporates from the film and leaves only the varnish that was previously in the pictorial layer;
the painting remains with its original varnish, without any damage as there was no friction, pressure, or penetration of the solvent into the structure of the work.
Conclusions
The use of a film containing ordered mesoporous silica (SBA-15) incorporated generated excellent results regarding the removal of the Paraloid B72™ varnish applied on works of art without damaging the underlying layers. The sheet soaked in the chosen solvent (xylene) acted on the surface of the different original artworks and collage mockup, dissolving and removing the varnish by adsorption, without causing abrasion to the paintings or damage to the butterfly wing; and, without penetrating the porous bodies of the works or causing various problems, such as, for example, retention or lifting of scales and/or elimination of low molecular weight molecules, formed in the aging process of the paintings, through the diffusion and dialysis of the solvent .
The sheet was able to carry a large amount of solvent and keep it on the surface of the work, at the sheet/work interface, adsorbed on the silica, letting it act slowly on the varnish, dissolving it due to the similarity in the solubility parameters of the solvent and solute, without, however, soaking the work. After dissolution of the varnish at the work/sheet interface, when the remainder of the solvent, which was adsorbed on the sheet, begins to evaporate, and leave the sheet, the solution (varnish + xylene) is transferred to the body of the sheet by silica by adsorption. In the third step, the solvent in the solution is evaporated, leaving the varnish dry inside the sheet. Then, the sheet can be removed from the work, which is clean, unvarnished, and undamaged.
This method is not intended to replace or reject any other method commonly used by restorers. It is just an alternative, one more tool that can be used or not when deciding on the cleaning of works of art. It should be understood that the sheet developed with SBA-15 silica was specifically used for the removal of Paraloid B72™ varnish from works of art with the xylene solvent. However, the application of cellulosic matrices incorporated with silica can be extended to remove any unwanted layer, provided that all materials and conditions of the system are studied. The large amount of silanol groups (Si-OH) on the surface of ordered mesoporous silica allows its functionalization with species that can confer greater hydrophilicity and hydrophobicity, that is, the material can be used for efficiently adsorbing hydrophilic and hydrophobic substances.
These adsorption sheets for cleaning works of art (removal of inappropriate materials: new or old varnishes, oxidized or not, inorganic and organic dirt, repainting, etc.) in restoration procedures are obtained with cellulose and highly ordered mesoporous silica pure, or functionalized and/or incorporated with chelating agents ranging from low dielectric constant (non-polar) to high dielectric constant (polar).
The choice of suitable material to be incorporated into the film must be made on the basis of tests carried out on site (pictorial layer and layer to be removed) and the expertise of the restorer. Adsorption sheets can dissolve the layer to be removed or soften it by decreasing the force of attraction between it and the pictorial layer. In this case, the softened layer can be removed mechanically using a swab.
It is very important to understand the physical-chemical nature and complexity of artwork surfaces before undertaking any cleaning procedure.
It is worth emphasizing that whatever the methodology chosen for restoration procedures, just as important as “what to do” is “how to do it”. It is clear that “what to do”, that is, what procedure and what materials should be used is paramount; however, the method of application is decisive for the success or not of the work. After the preliminary studies have been conducted and the method to be adopted has been decided, the knowledge of the restorer and skill will define the final results.
For example, the problem of abrasion of pictorial layers in the traditional cleaning method with a cotton swab rubbed over the surface or even residues left in the interstices of works where the cleaning method with gels or emulsions was used is due to the lack of dexterity and/or knowledge of the restorer (Khandekar, 2004). Likewise, cellulose film with silica must be used with knowledge: of the work, the layer to be removed, the suitable solvent, the film, and the process as a whole.
In the characterization of artwork materials used as a collage mockup, it was necessary to use and associate different techniques. Whenever possible, the techniques should be non-destructive or non-invasive. Otherwise, the experiments must be performed using amounts of samples in the milligram range. On the other hand, it must be considered that the work of scientific investigation (of works of art) does not dispense with historical, cultural, etc. investigations.
Author contributions
M.M. Rizzo and J.R. Matos participated in Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Writing - Original Draft, Supervision, Validation, Visualization, Writing - Review and Editing.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to thank the company MRIZZO Laboratório de Conservação e Restauração de Bens Culturais Ltda. for the use of space and loan of equipment; and the company Farma Service for loaning equipment.
References
American Society for Testing & Materials (2012). D3418-21 Standard Test Method for Transition Temperatures and Enthalpies of Fusion and Crystallization of Polymers by Differential Scanning Calorimetry. ASTM.
ASTM E1356-08 (2014). Standard Test Method for Assignment of the Glass Transition Temperatures by Differential Scanning Calorimetry. ASTM.
ASTM E1582-04 (2004). Standard Practice for Calibration of Temperature Scale for Thermogravimetry. ASTM.
Baij, L., Buijs, J., Hermans, J.J., Raven, L., Iedema, P. D., Keune, K. & Sprakel, J. (2020). Quantifying Solvent Action in Oil Paint Using Portable Laser Speckle Imaging. 10574. https://doi.org/10.1038/s41598-020-67115-1
Phenix, A., Wolbers, R. C., Townsend, J., Zumbühl, S., Bartoletti, A., Lee, J. & Ormsby, B. (2020). Removal of varnish: organic solvents as cleaning agents from: Conservation of Easel Paintings. Routledge. https://doi.org/10.4324/9780429399916-37
Masschelein-Kleiner, L. & Deneyer, J. (1981). Contribution à l'étude des solvants utilisés en conservation. In The International Council of Museums, ICOM Committee for Conservation [Proceedings]. 7th Triennial Meeting, Ottawa Canada..
Rizzo, M. M. (2015). Obtenção de filme adsorvente de celulose/SBA-15 para limpeza de superfícies policromadas em obras de arte.
Asunción, J. (2002). O Papel – Técnicas e Métodos Tradicionais de Fabrico. Ed. Estampa.
Bonini, Massimo, Lenz, Sebastian, Giorgi, Rodorico & Baglioni, Piero (2007). Nanomagnetic Sponges for the Cleaning of Works of Art. 23(17), 8681--8685. https://doi.org/10.1021/la701292d
Baglioni, P. (2007). Nanoscience for the Conservation of Cultural Heritage. In H. S. Garreau, Removal of Damaging Conservation Treatments on Mural Paintings (pp. 16-67).. Österbybruk.
Baglioni, P. (2010). Nanomagnetic Sponges for the Cleaning of Works of Art. 8681--8685.
Bonini, Massimo, Lenz, Sebastian, Giorgi, Rodorico & Baglioni, Piero (2007). Nanomagnetic Sponges for the Cleaning of Works of Art. 23(17), 8681--8685. https://doi.org/10.1021/la701292d
Barrett, E. P., Joyner, L. G. & Halenda, P. P. (1951). The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. 373--380. https://doi.org/10.1021/ja01145a126
Baglioni, P. (2010). Nanoscience for the Conservation of Cultural Heritage. Swedish National Heritage Board. 16-19.
Joyner, Leslie G., Barrett, Elliott P. & Skold, Ronald (1951). The Determination of Pore Volume and Area Distributions in Porous Substances. II. Comparison between Nitrogen Isotherm and Mercury Porosimeter Methods. 73(7), 3155-3158. https://doi.org/10.1021/ja01151a046
Chiantore, O. & Lazzari, M. (2001). Photo-oxidative stability of Paraloid acrylic protective polymers. 42(1), 17–27.
Dauchot-Dehon, M. (1975). Les effets de solvants sur les couches picturales. Alcools et acetone. In International Council of Museums, Bulletin [Anais]. 4th~Committee for Conservation Triennial Meeting Venice Italy 13-18 October. França.
Chiantore, O. & De Witte, E. (1978). Étude du temps de séchage du vernis Paraloid B72 sur les peintures.
Hansen, C.M. (1968). A mathematical description of film drying by solvent evaporation. 27-43.
Hansen, C.M. (1967). The three-dimensional solubility parameter, key to paint component affinities. 39(511), 104-117.
Khandekar, N. (2000). A Survey of the Conservation Literature Relating to the Development of Aqueous Gel Cleaning on Painted and Varnished Surfaces. 10-20.
Khandekar, N. (2002). Detection of Residues on the Surfaces of Objects Previously Treated with Aqueous Solvent Gels. 352-359.
Khandekar, N. (2004). Research into potential problems arising from the use of aqueous cleaning systems. Getty Publications. 12-17.
Khandekar, N. (2004). Research into Potential Problems Arising from the Use of Aqueous Cleaning Systems. Getty Conservation Institute. 5-17.
Machado, L. D. B. & Matos, J.R. (2004). Análise térmica diferencial e calorimetria exploratória diferencial. SV Técnicas de Caracterização de Polímeros. Artliber Ltda., São Carlos. 229-261.
Liliane Masschelein-Kleiner~ (1995). Ancient Binding Media Varnishes and Adhesives. ICCROM, Rome.
L. Masschelein-Kleiner (2004). Los Solventes. CNCR.
Masschelein-Kleiner, L, Heylen, J. & Tricot-Marckx, F. (1969). Contribution à l´analyse des liants, adhésives et vernis anciens. 75-82.
Masschelein-Kleiner, L., Heylen, J. & Tricot-Marckx, F. (1968). Contribution à l'analyse des liants, adhésifs et vernis anciens. 13(3), 105-121. https://doi.org/10.2307/1505316
Matos, J. R., Kruk, M., Mercuri, L. P., Jaroniec, M., Zhao, L., Kamiyama, T., Terasaki, O., Pinnavaia, T. J. & Liu, Y. (2003). Ordered mesoporous silica with large cage-like pores: structural identification and pore connectivity design by controlling the synthesis temperature and time. 821--829.
Matos, J. R., Mercuri, L. P., Kruk, M. & Jaroniec, M. (2001). Toward the synthesis of extra-large-pore MCM-41 analogues. 1726--1731.
Matos, J. R. & Machado, L. D. B. (2004). Análise térmica – termogravimetria. SV Técnicas de Caracterização de Polímeros. Artliber Ltda., São Carlos. 209-228.
Newman, D. J. & Nunn, C.J. (1975). Solvent retention in organic coatings. 222-243.
Ormsby, B., Learner, T., Foster, G., Druzik, J. & Schilling, M. (2007). Wet Cleaning Acrylic Emulsion Paint Films: An Evaluation of Physical, Chemical, and Optical Changes. The Getty Conservation Institute. 189–200.
Rizzo, M. M. (2015). Obtenção de filme adsorvente de celulose/SBA-15 para limpeza de superfícies policromadas em obras de arte.
J. Rouquerol, D. Avnir, C. W. Fairbridge, D. H. Everett, J. M. Haynes, N. Pernicone, J. D. F. Ramsay, K. S. W. Sing & K. K. Unger (1994). Recommendations for the characterization of porous solids (Technical Report). 66(8), 1739-1758. https://doi.org/doi:10.1351/pac199466081739
Schilling, M. R. (1989). The Glass Transition of Materials Used in Conservation. Maney Publishing. 34(3), 110-116.
Stavroudis, C. & Doherty, T. (2007). A Novel Approach to Cleaning II: Extending the Modular Cleaning Program to Solvent Gels and Free Solvents, Part 1. 29(3), 9-15.
Stavroudis, C., Doherty, T. & Wolbers, R. (2005). A New Approach to Cleaning I: Using Mixtures of Concentrated Stock Solutions and a Database to Arrive at an Optimal Aqueous Cleaning System. 27(2), 17-28.
Wolbers, R. (1994). Notes for the Workshop on New Methods in the Cleaning of Paintings. University of Delaware.
Wolbers, R. (1990). The use of a radio-isotopic assay for the direct measurement of residual cleaning materials on a paint film. IIC.
Richard C. Wolbers (1990). A RADIO-ISOTOPIC ASSAY FOR THE DIRECT MEASUREMENT OF RESIDUAL CLEANING MATERIALS ON A PAINT FILM. Routledge. 119-125. https://doi.org/10.1179/sic.1990.35.s1.025
Wolbers, R. (2000). Cleaning Painted Surfaces: Aqueous Methods. Archetype.
