Determination of Cu\(^{2+}\) and Zn\(^{2+}\) in Flaxseed Flour by FAAS and DPASV using Different Sample Treatments
Suquila, F. A. C.; Bertoldo, L. A.; Rocha, L. R.; Beal, A. Ferreira, M. P.; Ruiz, R. M.; Capelari, T.; Tarley, C. R. T.
DOI 10.5433/1679-0375.2023.v44.47941
Citation Semin., Ciênc. Exatas Tecnol. 2023, v. 44: e47941
Abstract:
The performance of three conventional treatments, including microwave-assisted acid digestion (MWAD), ultrasound-assisted extraction (UAE) and dry method (DAM) for determination of Cu\(^{2+}\) and Zn\(^{2+}\) in flaxseed meal, was evaluated. Quantification was performed by flame atomic absorption spectrometry (FAAS) and differential pulse anode stripping voltammetry (DPASV). The MWAD treatment was performed with both concentrated HNO\(_3\) (CA) and diluted acid (DA). The concentration of Cu\(^{2+}\) was determined by FAAS and DPASV, by different treatments, ranging from 14.4-26.0\(\mu\)g g\(^{-1}\) and 8.5-17.9\(\mu\)g g\(^{-1}\), respectively. The concentration of Zn\(^{2+}\) was possible only by FAAS (28.7-77.4\(\mu\)g g\(^{-1}\)). The highest concentrations were obtained using MWAD. The DAM showed values similar to MWAD for Cu\(^{2+}\), however, for Zn\(^{2+}\) it showed the lowest concentrations. UAE, in turn, showed low concentrations of Zn\(^{2+}\) in relation to MWAD, but similar results to MWAD-DA for Cu\(^{2+}\) in a sample with low fat content. Therefore, both the choice of treatment and the quantification technique play a crucial role in metal determination.
Keywords: linseed, functional food, voltammetry, sample preparation
Introduction
In the last years, great attention has been given to analysis of functional food, including mainly food from natural sources to supply energy and prevent diseases (Tonetta et al., 2017). In this regard, flaxseed is a source of lipids, fiber, protein and has a high content of minerals, such as copper (Cu\(^{2+}\)) and zinc (Zn\(^{2+}\)) (Cloutier et al., 2012; Kajla et al., 2015). Copper acts as an enzymatic cofactor to ceruloplasmin, assisting the iron oxidation and the hepatic markers’ mobility to bone marrow (Baierle et al., 2010). Zinc constitutes several enzymes and acts in vital functions, such as cellular division, genetic expression and transcription, cell membrane stabilizer, cognitive development, immunology, and the growing process.
Due to the importance of the copper and zinc intake, it is essential to determine these metals in food samples, especially those used as a functional food such as flaxseed flour. Nevertheless, for metal determination, the sample treatment has an important role to avoid errors, diminish time-consuming steps and to make the sample compatible to the analysis by the analytical techniques.
Thus, a satisfactory treatment should increase the availability of the analytes, avoiding interferences, loss of analytes, and contamination (Prestes et al., 2009). In order to choose an adequate sample treatment, the main parameters that must be considered are the nature of the sample and analyte, the concentration, and the detection technique (Korn et al., 2008).
Microwave-assisted acid digestion (MWAD) is widely used as an efficient sample treatment (Korn et al., 2008). This technique involves heating the sample in the presence of one or a mixture of concentrated acids, commonly, HNO\(_3\), HCl, HF, H\(_2\)SO\(_4\), and H\(_2\)O\(_2\) in closed flasks submitted to higher temperature and pressure (Korn et al., 2008; Arruda & Santelli, 1997). MWAD, compared with conventional acid digestion by convection heating in heating plate or digestion block, provides lower residual carbon and avoids loss of analyte by volatilization. In addition, due to higher temperature and pressure, diluted acids can be used for sample digestion with satisfactory results. However, this technique is still expensive for most laboratories.
The dry ashing method (DAM) using muffle furnace has also been used. The samples are added to crucible and digested by combustion at higher temperature in air atmosphere. This method is not indicated to volatile elements but, as higher mass of sample can be digested, low concentrations of elements can be determined (Morales-Rubio et al., 1992).
Ultrasound-assisted extraction (UAE) has also been used as sample treatment. Such method is environment-friendly because diluted acids are used, and lower analytical blanks can be obtained. Additionally, UAE is relatively easy to use, versatile, and requires low costs for implementation (Lemes & Tarley, 2021; Tiwari, 2015).
Atomic absorption spectrometry is considered one of the most analytical techniques used for metal determination. Among them, flame atomic absorption spectrometry (FAAS) is the most used, but due to its low sensitivity, this technique is more suitable for determining metal ions at higher concentration (mg L\(^{-1}\)), usually minerals in food samples (Subramanian et al., 2012).
Electroanalytical methods have been used successfully during years for metal ions determination with higher sensitivity compared with FAAS (Corazza et al., 2020). Differential pulse anodic stripping voltammetry (DPASV) is widely used for copper and zinc determination. The method is commonly based on a preconcentration step at surface of mercury electrode as working electrode by applying a negative potential (cathodic region). The reduction of the target metal promotes an amalgam formation onto a mercury electrode, according to equation (1) given by
(1)
\[ M+n + {ne}^{-} \rightleftharpoons M\left(Hg\right).\]
Upon preconcentration step, a stripping step occurs in an anodic direction, equation (2),
(2)
\[ M\left(Hg\right) \rightleftharpoons M+n + {ne}^{-} +Hg\]
and a voltammogram is registered, in which the peak current is proportional to the metal concentration (Oliveira et al., 2004; Raj et al., 2013).
Considering the large use of flaxseed as a functional food; the complexity of sample, which contains high amount of fat and protein; the importance of sample treatments and their performance on the analytical techniques, this paper deals with a comparative study involving MWAD (using concentrated and diluted acid), DAM and UAE as sample treatment methods of commercial flaxseed flour and their influence on the Cu\(^{2+}\) and Zn\(^{2+}\) determination by FAAS and DPASV.
Materials and methods
Materials
All reagents used were of analytical grade, and all working solutions were prepared in ultrapure water (18.2 M\(\Omega\) cm) from an ELGA PURELAB® Maxima purification system (High Wycombe, Bucks, UK). To avoid contamination, all glassware and storage bottles were kept in a 10.0% v/v nitric acid solution for 24 h. Two commercial brands of brown flaxseed flour were purchased at the supermarket, named in this study as sample 1 (S1 -Jasmine) and sample 2 (S2 - Vitao Linha Chef). It must be pointed out that the samples were used as sold, and were not water washed, ground, and sieved due to high content of fat.
The working solutions of Cu\(^{2+}\) and Zn\(^{2+}\) were prepared from a standard solution of concentration 1000 mg L\(^{-1}\) from Quimilab® (Jacareí, SP, Brazil). Analytical grade hydrogen peroxide (H\(_2\)O\(_2\), 30.0% v/v) from Synth (Diadema, SP, Brazil) and nitric acid (HNO\(_3\), 65.0% w/w) from Sigma-Aldrich (Steinheim, Germany), sodium acetate buffer solution (CH\(_3\)COONa, 99.0%) was prepared from Vetec (Rio de Janeiro, RJ, Brazil), mercury (II) chloride (HgCl\(_2\), 99.0%) from Merck. The pH of the working solutions was adjusted with sodium hydroxide (NaOH, 99.0%) and hydrochloric acid (HCl, 37.0%) from Vetec and Panreac (Darmstadt, Germany), respectively.
Instrumentation
A microwave digestion system Milestone Inc® model ETHOS One (Sorisole, Italy) was used for acid digestion, while the Cu\(^{2+}\) and Zn\(^{2+}\) leaching from the samples was performed with an ultrasound bath Quimis® model Q335D2 (Diadema, SP, Brazil). A muffle furnace from Fornitec Ind. and Com. Ltda® (São Paulo, SP, Brazil) was used in the dry ashing method. The quantification of Cu\(^{2+}\) and Zn\(^{2+}\) was carried out using a flame atomic absorption spectrometer (FAAS) model AA-6601F Shimadzu® (Kyoto, Japan). The composition of the flame was air/acetylene with a flow rate of 15.0 and 2.0 mL min\(^{-1}\), respectively. Hollow cathode lamps (Plainview, NY, EUA) were used as radiation source and operated at 10.0 mA, with wavelengths of 324.75 and 213.86 nm for Cu\(^{2+}\) and Zn\(^{2+}\), respectively. For background correction, a deuterium lamp was employed.
Differential pulse anodic stripping voltammetry (DPASV) was also used for Cu\(^{2+}\) and Zn\(^{2+}\) quantification using a potentiostat/galvanostat Palm Instruments BV® PalmSens (Houten, Netherlands), controlled by software Palm Instruments BV® PSTrace 4.4 (Houten, Netherlands) in a single-compartment electrochemical cell, containing an Ag/AgCl (KCl 3.0 mol L\(^{-1}\)) as a reference electrode, a platinum wire as an auxiliary electrode and a glassy carbon electrode (Metrohm, Herisau, Switzerland) modified with mercury film as a working electrode. All measurements were made with a fixed volume of 10.0 mL.
The pH measurements were performed with the pH-meter Metrohm® 827 pH lab. A peristaltic pump Gilson® Minipuls Evolution (Middleton, Wi, USA) with Tygon® tubes (Courbevoie, France) was coupled to the ultrasound bath in order to recirculate the water and keep constant the bath water temperature.
Microwave-assisted digestion (MWAD)
The influence of concentrated and diluted HNO\(_3\) on the digestion process was evaluated. Therefore, 400.0 mg of each sample was weighed and transferred to the reaction flask and subjected to microwave-assisted digestion with concentrated acid (MWAD-CA), adding 8.0 mL of HNO\(_3\) (65.0%) and 2.0 mL of H\(_2\)O\(_2\) (30.0%) or with dilute acid (MWAD-DA), using 2.0 mL of HNO\(_3\) (65.0%), 0.5 mL of H\(_2\)O\(_2\) (30.0%) and 7.5 mL of H\(_2\)O.
The heating program of microwave oven was carried out in four steps, where:
Step 1: heating to 120°C for 5 min (750W);
Step 2: plateau at 120 °C for 2 min (750W);
Step 3: heating to 200 °C for 10 min (1200W);
Step 4: plateau at 200 °C for 15 min (1200W),
with exhaustion time of 30 min. Upon this time, digested samples were transferred to a 25.0 mL volumetric flask, and the volume was made up with ultrapure water.
The samples were stored in polyethylene bottles and refrigerated until the analysis. The MWAD method was based on literature (Lemes & Tarley, 2021) with minor modifications.
Decomposition by dry ashing method (DAM)
1.00 g of each sample was weighed in a porcelain crucible. Then, the sample was burned in a Bunsen burner until carbonization. Afterward, the crucible containing the reduced samples was placed in a muffle furnace at 500 °C for 10 h. The obtained ash was dissolved in 1.0 mol L\(^{-1}\) of HNO\(_3\), transferred to a 25.0 mL volumetric flask, and the volume was made up with ultrapure water. The samples were stored and kept under refrigeration until the moment of analysis. The DAM method was based on reference (Krug & Rocha, 2016).
Ultrasound-assisted extraction (UAE)
The procedure for leaching Cu\(^{2+}\) and Zn\(^{2+}\) via ultrasound was carried out by mixing 400.0 mg of sample with 10.0 mL of a mixture of HCl, HNO\(_3\) and H\(_2\)O\(_2\), whose final concentrations were 4.0 mol L\(^{-1}\), 4.0 mol L\(^{-1}\) and 0.5 mol L\(^{-1}\), respectively, in a 50.0 mL tube Falcon®. Using a homemade support with thirteen holes, see Figure 1, the sample were placed in the ultrasound and kept for 1 h. A preliminary study was carried out to evaluate the efficiency of each position of the support, and all thirteen positions indicated similar extraction efficiency. The ultrasound bath consisted of 2.0 L of distilled water containing 0.2% (v/v) detergent. As already mentioned, the peristaltic pump was operated at flow rate of 14.0 mL min\(^{-1}\) to recirculate the water and keep constant the bath water temperature (Capelo et al., 2005; Lorimer & Mason, 1987; Shirsath et al., 2012).
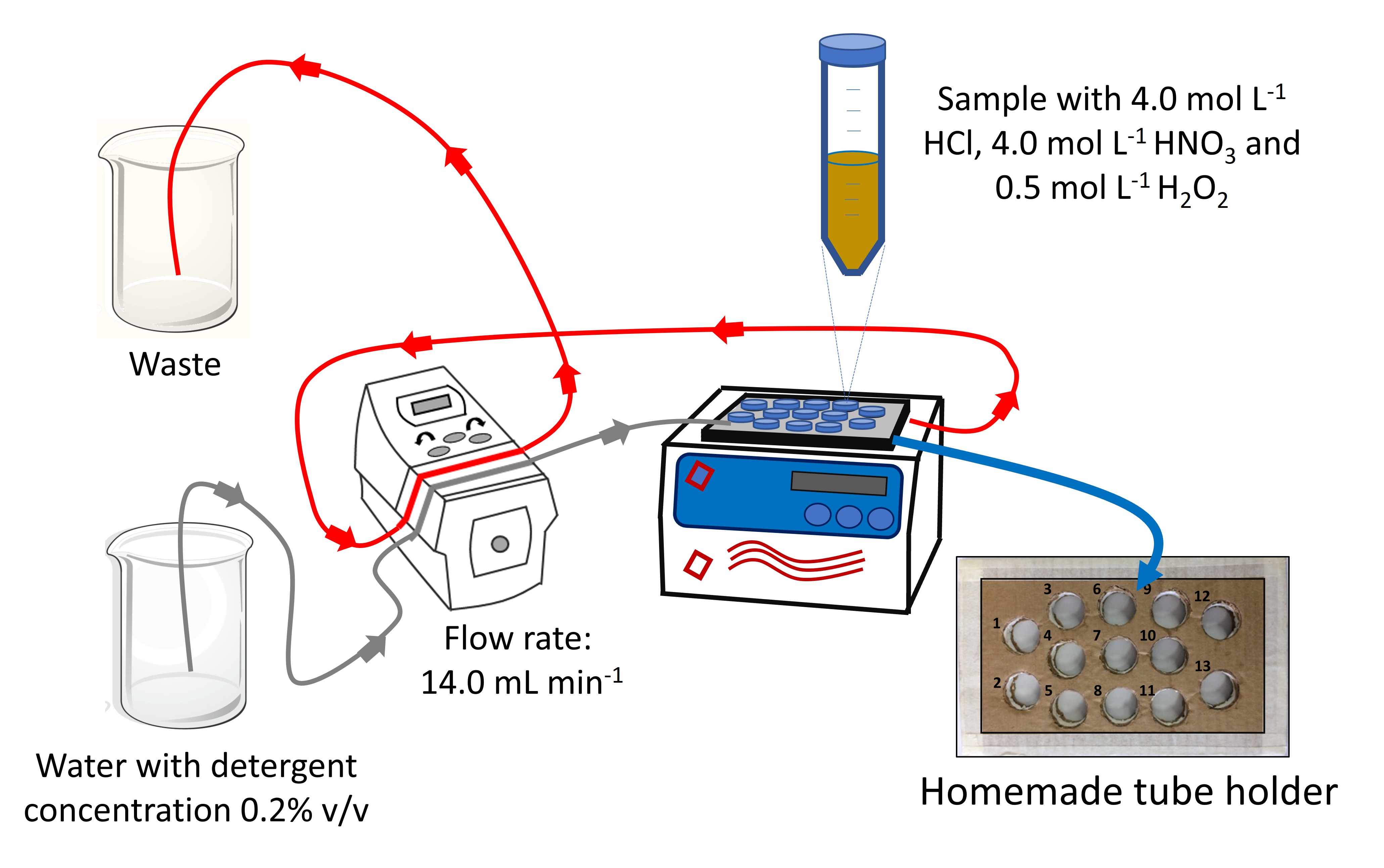
After extraction time, the tubes were centrifuged for 5 min, and 7.0 mL of the supernatant was removed with the aid of a micropipette and added to a volumetric flask of 25.0 mL, and the volume was made up with ultrapure water. The samples were stored and kept under refrigeration and then analyzed immediately.
Sensor preparation and voltammetric analysis
The electrochemical deposition of mercury film on the surface of glassy carbon electrode was performed in situ in the presence of Cu\(^{2+}\) and Zn\(^{2+}\) in similar way as reported in the literature (Gorla et al., 2015; Oliveira et al., 2004). Electrodeposition was carried out in 0.15 mol L\(^{-1}\) acetate buffer (CH\(_3\)COO\(^-\)/CH\(_3\)COOH) at pH 4.0 containing 1.0 \(\times\) 10\(^{-5}\) mol L\(^{-1}\) of Hg\(^{2+}\) in the electrochemical cell. A potential of -1.3 V during 180 s was applied to electrochemically reduce metal ions under magnetic stirring. Upon electrodeposition and an equilibrium time of 2 s, the measurements were carried out by stripping voltammetry toward anodic region from -1.2 V to 0.0 V using differential pulse voltammetry (DPV), with a scan rate of 100 mV s\(^{-1}\), amplitude and pulse time of 80 mV and 5 ms, respectively. Therefore, DPASV was also evaluated to determine Cu\(^{2+}\) and Zn\(^{2+}\).
The volumes of treated samples added to electrochemical cell for the respective procedures MWDA, DAM, and UAE procedures were 600.0 \(\mu\)L, 250.0 \(\mu\)L and 800.0 \(\mu\)L. The DPASV measurements were carried out in electrochemical cell containing 10.0 mL of acetate buffer (CH\(_3\)COO\(^-\)/CH\(_3\)COOH) at pH 4.0 as supporting electrolyte.
Results and discussions
Analytical curve and sample treatment methods comparison
Initially, analytical curves were obtained using FAAS ranging from 50 to 2000 ug L\(^{-1}\) for Cu\(^{2+}\) and 100 to 1700 \(\mu\)g L\(^{-1}\) for Zn\(^{2+}\). The linear equations found were equations (3) and (4):
(3)
\[ Abs = 0.0001[\mathrm{Cu}^{2+}] + 0.0321~~(\mathrm{R}^2 = 0.999),\]
(4)
\[ Abs = 0.0004[\mathrm{Zn}^{2+}] + 0.0223~~(\mathrm{R}^2 = 0.999).\]
For DPASV the linear range was ranged from 4.9 to 29.0 for Cu\(^{2+}\) and 20.0 to 323.0 \(\mu\)g L\(^{-1}\) for Zn\(^{2+}\) giving rise to respective linear equations given by equations (5) and (6):
(5)
\[ (\mu A)_{\mathrm{current}} = 0.8379[\mathrm{Cu}^{2+}] - 2.5371 (\mathrm{R}^2 = 0.992),\]
(6)
\[ (\mu A)_{\mathrm{current}}= 0.0540[\mathrm{Cu}^{2+}] - 2.8032 (\mathrm{R}^2 = 0.975).\]
The anodic potential of Cu\(^{2+}\) and Zn\(^{2+}\) were found to be -0.2 and -1.1 V, respectively.
The results of Cu\(^{2+}\) and Zn\(^{2+}\) in flaxseed flour obtained by FAAS and DPASV using the different sample treatments are shown in Table 1 and 2.
| Cu\(^{2+}\) (\(\mu\)g g\(^{-1}\)) | |||||
| Technique | MWAD-CA* | MWAD-DA* | DAM* | UAE* | |
| FAAS | S1 | 14.2\(^b\) \(\pm\) 0.5 | 17.2 \(^a\) \(\pm\) 0.42 | 15.1a \(\pm\) 0.8 | 14.7\(^a\) \(\pm\) 1.9 |
| S2 | 22.4\(^b\) \(\pm\) 0.5 | 26.6 \(^a\) \(\pm\) 0.4 | 24.7\(^a\) \(\pm\) 2.9 | 17.9c \(\pm\) 1.9 | |
| DPASV | S1 | 9.4\(^a\) \(\pm\) 2.9 | 8.5\(^a\) \(\pm\) 2.8 | 12.2\(^a\) \(\pm\) 3.2 | 7.5\(^a\) \(\pm\) 0.3 |
| S2 | 12.9\(^a\) \(\pm\) 2.3 | 15.9\(^a\) \(\pm\) 3.5 | 17.9\(^a\) \(\pm\) 3.9 | 6.2\(^b\) \(\pm\) 0.2 | |
*MWAD-CA: microwave-assisted acid digestion with concentrated acid;
*MWAD-DA: microwave-assisted acid digestion with diluted acid;
*DAM: Dry Ashing Method;
*UAE: ultrasound-assisted extraction.
Different horizontal letters indicate statistical difference by the student’s t-test at the 95% confidence level.
| Zn\(^{2+}\) (\(\mu\)g g\(^{-1}\)) | |||||
| Technique | MWAD-CA* | MWAD-DA* | DAM* | UAE* | |
| FAAS | S1 | 52.5\(^a\) \(\pm\) 0.6 | 53.6\(^a\) \(\pm\) 0.5 | 28.7\(^c\) \(\pm\) 1.3 | 45.3\(^b\) \(\pm\) 0.6 |
| S2 | 77.4\(^a\) \(\pm\) 1.0 | 67.5\(^b\) \(\pm\) 0.6 | 36.6\(^d\) \(\pm\) 0.4 | 52.8\(^c\) \(\pm\) 2.4 | |
*MWAD-CA: microwave-assisted acid digestion with concentrated acid;
*MWAD-DA: microwave-assisted acid digestion with diluted acid;
*DAM: Dry Ashing Method;
*UAE: ultrasound-assisted extraction.
Different horizontal letters indicate statistical difference by the student’s t-test at the 95% confidence level.
As one can see from Table 1, for Cu\(^{2+}\), higher concentrations were observed using FAAS compared with DPASV. This result was observed either for S1 as S2 sample, which clearly indicates, as expected, that DPASV is more prone to interferences from the matrices than FAAS. Such statement is more evident for Zn\(^{2+}\) determination, where its determination was not possible by DPASV even using MWAD-DA method as sample treatment, see Figure 2.
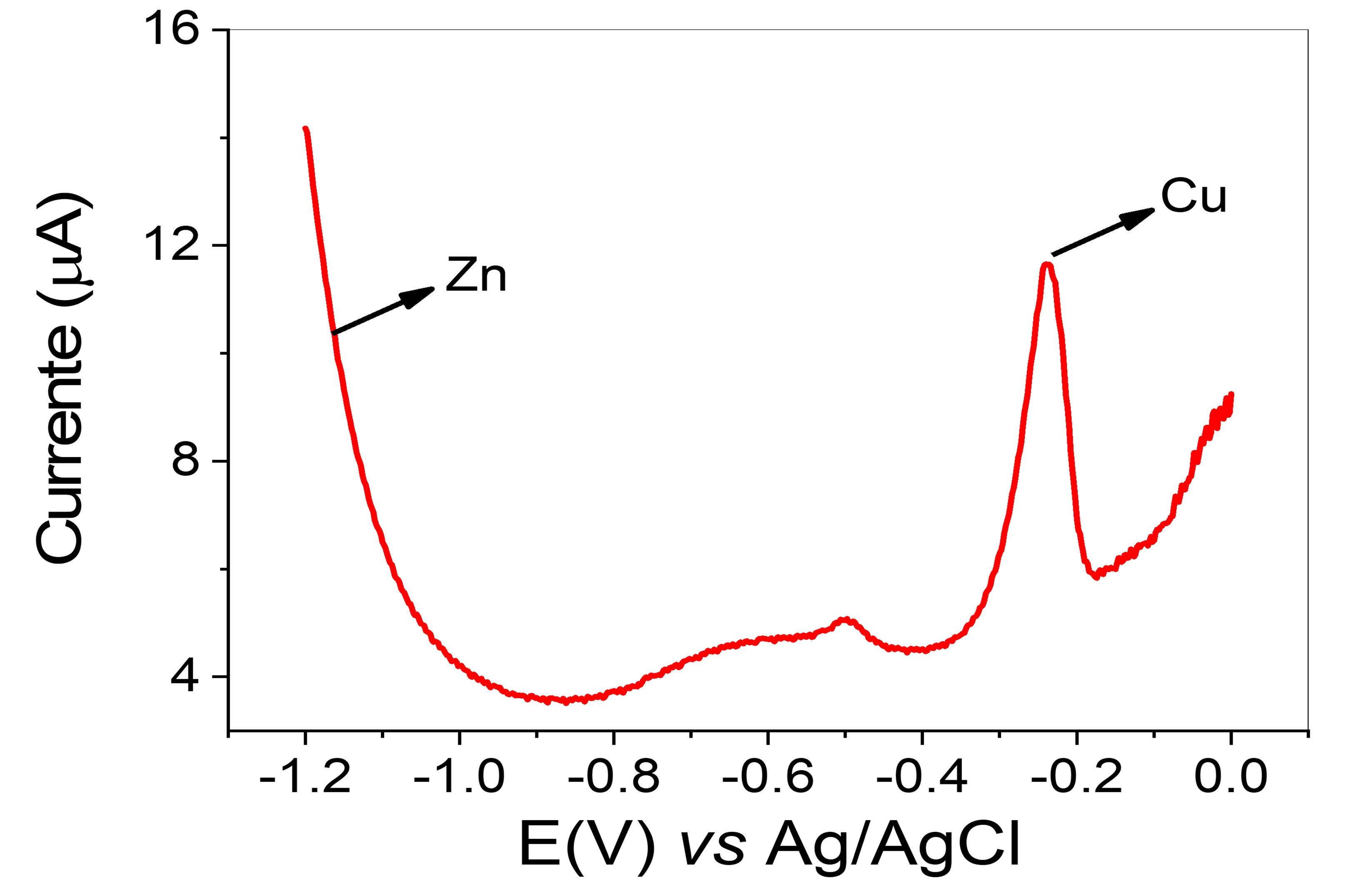
One should note that detectability for Zn\(^{2+}\) using DPASV is lower than Cu\(^{2+}\). Therefore, some attempts were carried out for Zn detecting. The time of electrochemical deposition was increased from 180 to 900 s and the sample was evaporated and retaken in supporting electrolyte to diminish the sample acidity. Even using these procedures no analytical signal for Zn\(^{2+}\) was observed. Thus, the interference on the Zn\(^{2+}\) determination may be explained, in fact, from matrices effect and the formation of intermetallic compounds formed during electrochemical deposition, such as copper:zinc (1:3) (Shuman & Woodward, 1976).
An important issue regarding the Cu\(^{2+}\) determination by DPASV are the higher standard deviations of measurements compared with FAAS, which is intrinsic of technique and again dependent on the sample matrices.
From Table 1, it is possible to evaluate the influence of concentrated and diluted acid in the microwave-assisted digestion procedure for metals determination by FAAS. As observed, the concentrations of Cu\(^{2+}\) in both samples (S1 and S2) and Zn\(^{2+}\) in S1 were higher when using diluted acid. Several studies demonstrated the higher digestion efficiency using diluted nitric acid and H\(_2\)O\(_2\) due to formation of peroxynitrite and acid regeneration along the process (Araújo et al., 2002; Bendicho et al., 2010; Gonzalez et al., 2009; Kingston & Haswell, 1997; Muller et al., 2016).
The various reactions in nitric acid regeneration in closed reaction flasks during microwave digestion can be demonstrated. Upon organic matter oxidation with diluted nitric acid, NO\(_{2(\mathrm{g})}\) is produced, equation (7), which is absorbed in the solution and reacts with water to form HNO\(_{3(\mathrm{aq})}\) and HNO\(_{2(\mathrm{aq})}\), equation (8):
(7)
\[\begin{gathered} \label{7} \left({\mathrm{CH}}_2\right)_\mathrm{n}+\mathrm{4HNO}_{3\left(\mathrm{conc}\right)} \\ \rightarrow \mathrm{CO}_{2 \left(\mathrm{g}\right)} +\mathrm{4NO}_{2\left(\mathrm{g}\right)}+ {2\mathrm{H_2O}}_{\left(\mathrm{l}\right)}, \end{gathered}\]
(8)
\[ 2\mathrm{NO}_{2\left(\mathrm{g}\right)}\ + {\mathrm{\mathrm{H_2O}}}_{\ \left(\mathrm{l}\right)}\ \rightarrow\ \mathrm{HNO}_{3\left(\mathrm{aq}\right)} + \mathrm{HNO}_{2\left(\mathrm{aq}\right)}.\]
The acid HNO\(_{2(\mathrm{aq})}\) decomposes into NO\(_{(\mathrm{g})}\) and NO\(_{2(\mathrm{g})}\), equation (9),
(9)
\[ \mathrm{HNO}_{2\left(\mathrm{aq}\right)}\ \rightarrow\ {{\mathrm{H_2O}}}_{\ \left(\mathrm{l}\right)}\ +\ \mathrm{NO}_{2\left(\mathrm{g}\right)}\ + \mathrm{NO}_{\left(\mathrm{g}\right)},\] while the HNO\(_{3(\mathrm{aq})}\) restarts the oxidation cycle. It is known that the main decomposition product of concentrated nitric acid is NO\(_{2(\mathrm{g})}\), equation (7), and in dilute nitric acid solutions, there is a preferential production of NO\(_{(\mathrm{g})}\). In this case, during the oxidation of the organic matter by diluted nitric acid, the NO\(_{(\mathrm{g})}\) produced, equation (9) reacts with the O\(_{2(\mathrm{g})}\) from H\(_2\)O\(_2\) decomposition to form NO\(_{2(\mathrm{g})}\), equation (10), given by
(10)
\[ \mathrm{2NO}_{\left(\mathrm{g}\right)}\ +\ \mathrm{O}_{2\ \left(\mathrm{g}\right)}\rightarrow\ 2\mathrm{NO}_{2\left(\mathrm{g}\right)}.\]
Thus, the use of H\(_2\)O\(_2\) also contributes to maintaining the efficiency of decomposition, acting as a source of O2 and oxidant of organic matter (Muller et al., 2016). Then, the formed NO\(_{2(\mathrm{g})}\) is reabsorbed in the liquid phase, leading to the production of HNO\(_3\)(aq) and HNO\(_{2(\mathrm{aq})}\), equation (4), continuing the reactions presented in the equations (3)-(5). Thus, it is possible to use diluted nitric acid to digest organic samples without decreasing the decomposition efficiency. The nitric acid regeneration cycle ends when all the oxygen is consumed.
The concentrations of Cu (sample S1) obtained by FAAS using DAM and UAE methods were statistically equal to MWAD-DA method (t-test with a 95% confidence interval); however, for S2 there was statistical difference between UAE and MWAD-DA. Such outcome can be explained due to higher content of total fat in S2 (40%) samples compared with S1 (34%), which makes the UAE less efficient for leaching Cu from the sample.
With regard the Zn\(^{2+}\) determination by FAAS, Table 2, the higher concentrations were obtained using MWAD-CA and MWAD-DA. The results for sample S1 were statistically equal using diluted and concentrated acid, but higher Zn\(^{2+}\) concentration was achieved for S2 using microwave-assisted digestion in concentrated acid. As mentioned, S2 sample has higher fat content, which requires higher acid concentration for better digestion efficiency.
Notably, DAM method furnished the lower Zn concentration, whose result can be rationalized bearing in mind the formation of zinc chloride (ZnCl\(_2\)) during the decomposition by DAM, favoring the volatilization of Zn\(^{2+}\) during the procedure (Krug & Rocha, 2016).
Thus, DAM has some limitations in the determination of the Zn element, despite being a simpler sample preparation method. On the other hand, MWAD is an exhaustive approach that offers several advantages, such as precise power control, reduced analysis time, smaller amount of sample required, minimization of contamination, no loss of analytes, and being able to analyze several samples at once. These advantages result in an improved efficiency of the digestion process (Arruda & Santelli, 1997).
Although the use of the standard addition method in certain cases can minimize the matrix effect, it is important to emphasize that this strategy has some disadvantages, such as the need for a greater total amount of sample. In this context, it is important to consider that this approach would not be beneficial to improve the results obtained by DAM, especially taking into account the analytical frequency.
The results for Zn\(^{2+}\) concentration obtained by UAE were statistically different regarding MWAD-CA and MWAD-DA. It is very well known that ultrasonic extraction efficiency depends upon homogeneity of sample, particle size, ultrasonic time extraction and acid concentration (Suquila et al., 2019; Beal et al., 2018). Thus, it is clearly demonstrated that for the successful of UAE, an adequate optimization must be carried out bearing in mind the nature of sample and analyte.
The highest concentrations of Cu\(^{2+}\) and Zn\(^{2+}\) in flaxseed flour were determined by FAAS and using MWAD, mainly using diluted acid. Similar values to this study were found in the literature, e.g., Hussain et al., 2008 who reported concentrations ranging from 19 to 34.5 \(\mu\)g g\(^{-1}\) and 44.3 to 78.6 \(\mu\)g g\(^{-1}\) for Cu\(^{2+}\) and Zn\(^{2+}\), respectively.
Conclusions
The influence of four sample treatments MWAD-CA, MWAD-DA, UAE, and DAM on the Cu\(^{2+}\) and Zn\(^{2+}\) determination by FAAS and DPASV was herein reported. It was observed that DPASV furnished the lower Cu concentrations in the samples S1 and S2 regarding FAAS using the four sample treatments, indicating the greatest susceptibility to matrices interferences, as also noticed by absence of analytical signal for Zn\(^{2+}\).
In the overall, MWAD-DA was the most efficient sample treatment for Cu\(^{2+}\) and Zn\(^{2+}\) determination by FAAS. DAM presented similar performance of MWAD-DA for Cu\(^{2+}\) determination, but low efficiency for Zn\(^{2+}\), as result of volatile species formed during procedure. Although the UAE method is environment-friendly it was demonstrated that a careful optimization of variables that play an important role on the ultrasonic extraction must be performed for producing reliable results.
Author contributions
F. A. C. Suquila, L. R. Rocha, A. Beal, R. M. Ruiz, and T. Capelari participated in the Conceptualization, Data Curation, Formal Analysis, Investigation. L. A. Bertoldo and M. P. Ferreira: Conceptualization, Formal Analysis, Writing – original draft. C. R. T. Tarley : Conceptualization, Supervision, Methodology, Validation, Visualization, Writing – revision and editing.
Conflicts of interest
The authors certify that there is not a commercial or associative interest that represents a conflict of interest in relation to the manuscript.
Acknowledgments
The authors acknowledge the financial support and fellowships of Coordenação de Aperfeiçoamento de Nível Superior (CAPES) (Project Pró-Forenses 3353/2014 Grant no. 23038.007082/2014-03), Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grant No 307432/2017-3, 307505/2021-9, 420097/2021-0, 190434/2017-1 and 311113/2019-2), SETI do Paraná, and Instituto Nacional de Ciência e Tecnologia de Bioanalítica (INCT) (FAPESP Grant No 2014/50867-3 and CNPq Grant No 465389/2014-7).
References
Korn, M. G. A., BoaMorte, E. S., Santos, D. C. M. B., Castro, J. T., Barbosa, J. T. P., Teixeira, A. P., Fernandes, A. P., Welz, B., Santos, W. P. C., Santos, E. B. G. N., & Korn, M. (2008). Sample Preparation for the Determination of Metals in Food Samples Using Spectroanalytical Methods—A Review. Taylor & Francis. 43(2), 67-92. https://doi.org/10.1080/05704920701723980
Araújo, G. C. L., Gonzalez, M. H., Ferreira, A. G., Nogueira, A. R. A., & Nóbrega, J. A. (2002). Effect of acid concentration on closed-vessel microwave-assisted digestion of plant materials. 57(12), 2121-2132. https://doi.org/https://doi.org/10.1016/S0584-8547(02)00164-7
Arruda, M. A. Z., & Santelli, R. E. (1997). Mechanization in sample preparation by microwares: the state-of-the-art. 20(638), 638--643. https://doi.org/10.1590/S0100-40421997000600012
Baierle, M., Valentini, J., Paniz, C., Moro, A., Barbosa Junior, F., & Garcia, S. C. (2010). Possible effects of blood Copper on hematological parameters in elderly. 46(463), 180--186. https://doi.org/10.1590/S1676-24442010000600006
Beal, A., Almeida, F. G., Moreira, C. A. B., Santos, I. M., Curti, S. M. M., Martins, L. D.,& Tarley, C. R. T. (2018). A new analytical method for lead determination in atmospheric particulate matter by a combination of ultrasound-assisted extraction and supramolecular solvent preconcentration. 3745--3753. https://doi.org/10.1039/C8AY01092G
Bendicho, C., Pena, F., Costas, M., Gil, S., & Lavilla, I. (2010). Photochemistry-based sample treatments as greener approaches for trace-element analysis and speciation. 29(7), 681--691. https://doi.org/10.1016/j.trac.2010.05.003
Capelo, J. L., Maduro, C., & Vilhena, C. (2005). Discussion of parameters associated with the ultrasonic solid-liquid extraction for elemental analysis (total content) by electrothermal atomic absorption spectrometry: an overview. 12(3), 225--232. https://doi.org/10.1016/j.ultsonch.2003.10.010
Cloutier, S., Ragupathy, R., Miranda, E., Radovanovic, N., Reimer, E., Walich-nowski, A., Ward, K., Rowland, G., Duguid, S., & Banik, M. (2012). Integrated consensus genetic and physical maps of flax (Linum usitatissimum L.). 125(1), 1783--1795. https://doi.org/10.1007/s00122-012-1953-0
Corazza, M. Z., Santos, P. M., Segatelli, M. G., Pereira, A. C., & Tarley, C. R. (2020). Avaliação de nanotubos de carbono funcionalizados visando o desenvolvimento de métodos de pré-concentração de íons metálicos e determinação por técnicas e eletroanalíticas. 43(8), 1086--1103. https://doi.org/10.21577/0100-4042.20170583
Oliveira, M. F., Saczk, A. A., Okumura, L. L., Fernandes, A. P., Moraes, M., & Stradiotto, N. R. (2004). Simultaneous determination of zinc, copper, lead, and cadmium in fuel ethanol by anodic stripping voltammetry using a glassy carbon-mercury-film electrode. 380(380), 135--140. https://doi.org/10.1007/s00216-004-2733-8
Gorla, F. A., de Oliveira, F. M., Duarte, E. H., de Mattos, A. E., da Silva, E. T., Galão, O. F., & Tarley, C. R. T. (2015). Sensor de pasta de nanotubos de carbono modificado com filme de bismuto para determinação de íons metálicos em etanol combustível. 36(1), 41--50. https://doi.org/10.5433/1679-0375.2015v36n1p41
Gonzalez, M. H., Souza, G. B., Oliveira, R. V., Forato, L. A., Nóbrega, J. A., & Nogueira, A. R. A. (2009). Microwave-assisted digestion procedures for biological samples with diluted nitric acid: Identification of reaction products. 79(2), 396--401. https://doi.org/10.1016/j.talanta.2009.04.001
Hussain, S., Anjum, F., Butt, M., & Sheikh, M. (2008). Chemical compositions and functional properties of flaxseed flour. 649-653.
Kajla, P., Sharma, A., & Sood, D. R. (2015). Flaxseed-a potential functional food source. 1857. https://doi.org/10.1007/s13197-014-1293-y
Kingston, H. M., & Haswell, S. J. (1997). Microwave-Enhanced Chemistry. Fundamentals, Sample Preparation, and Applications. American Chemical Society.
Krug, F. J., & Rocha, F. R. P. (2016). Métodos de Preparo de Amostras para Análise Elementar. Sociedade Brasileira de Química; Edit. SBQ..
Lemes, L. F. R., & Tarley, C. R. T. (2021). Combination of supramolecular solvent-based microextraction and ultrasound-assisted extraction for cadmium determination in flaxseed flour by thermospray flame furnace atomic absorption spectrometry. 129695. https://doi.org/10.1016/j.foodchem.2021.129695
Lorimer, J. P. & Mason, T. J. (1987). Sonochemistry. Part 1-The physical aspects. 6(16), 239--274. https://doi.org/10.1039/CS9871600239
Mason, T. J. (1991). Practical Sonochemistry: User’s guide to application in chemistry and chemical engineering. Chichester.
Morales-Rubio, A., Salvador, A. & de la Guardia, M. (1992). Microwave muffle furnace assisted decomposition of vegetable samples for flame atomic spectrometric determination of Ca, Mg, K, Fe, Mn and Zn. 452--456. https://doi.org/10.1007/BF00322206
Muller, E. I., Souza, J. P., Muller, C. C., Muller, A. L. H., Mello, P. A., & Bizzi, C. A.(2016). Microwave-assisted wet digestion with H2O2 at high temperature and pressure using single reaction chamber for elemental determination in milk powder by ICP-OES and ICP-MS. 156--157. https://doi.org/10.1016/j.talanta.2016.05.019
Prestes, O. D., Friggi, C. A., Adaime, M. B., & Zanella, R. (2009). QuEChERS: um método moderno de preparo de amostra para determinação multirresíduo de pesticidas em alimentos por métodos cromatográficos acoplados à espectrometria de massas. 32(6), 1620. https://doi.org/10.1590/S0100-40422009000600046
Raj, J., Raina, A., & Dogra, T. D. (2013). Zin (Zn) analysis in milk by microwave oven digestion and differential pulse anodic stripping voltammetry (DPASV) technique. 39007.
Shirsath, S. R., Sonawane, S. H., & Gogate, P. R. (2012). Scaling up the extraction of natural products using ultrasonic irradiation - a review of the current state. 10--23. https://doi.org/10.1016/j.cep.2012.01.001
Shuman, M. S. & Woodward, Jr., G. P. (1976). Intermetallic compound formation between copper and zinc in mercury and its effects on anodic stripping voltammetry. 1979.
Subramanian, R., Gayathri, S., Rathnavel, C., & Raj, V. (2012). Analysis of mineral and heavy metals in some medicinal plants collected from local market. S74--S78. https://doi.org/10.1016/S2221-1691(12)60133-6
Suquila, F. A. C., Scheel, G. L., Oliveira, F. M., & Tarley, C. R. T. (2019). Assessment of ultrasound-assisted extraction combined with supramolecular solvent-based microextraction for highly sensitive cadmium determination in medicinal plant sample by TS-FF-AAS. 1071--1077. https://doi.org/10.1016/j.microc.2018.12.011
Tiwari, B. K. (2015). Ultrasound: A clean, green extraction technology. 100--109. https://doi.org/10.1016/j.trac.2015.04.013
Tonetta, V., Dambrós, B. P., Minotto, E., & Santin, N. C. (2017). O papel da linhaça como agente redutor de colesterol e perda de peso. 11(63), 159--167.
